Plasmids, a molecular cornerstone of antimicrobial resistance in the One Health era
IF 69.2
1区 生物学
Q1 MICROBIOLOGY
引用次数: 3
Abstract
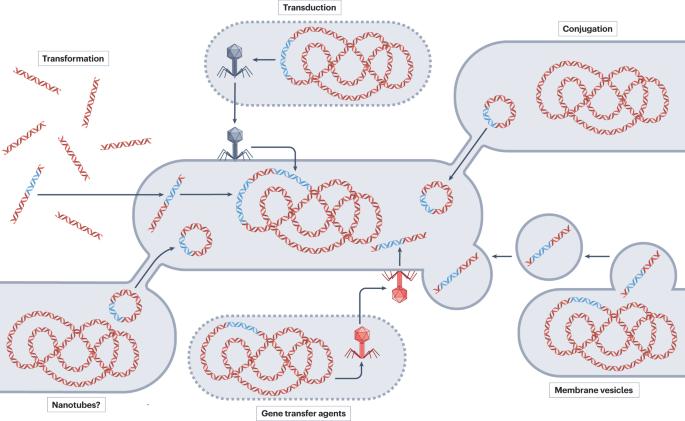
Antimicrobial resistance (AMR) poses a substantial threat to human health. The widespread prevalence of AMR is, in part, due to the horizontal transfer of antibiotic resistance genes (ARGs), typically mediated by plasmids. Many of the plasmid-mediated resistance genes in pathogens originate from environmental, animal or human habitats. Despite evidence that plasmids mobilize ARGs between these habitats, we have a limited understanding of the ecological and evolutionary trajectories that facilitate the emergence of multidrug resistance (MDR) plasmids in clinical pathogens. One Health, a holistic framework, enables exploration of these knowledge gaps. In this Review, we provide an overview of how plasmids drive local and global AMR spread and link different habitats. We explore some of the emerging studies integrating an eco-evolutionary perspective, opening up a discussion about the factors that affect the ecology and evolution of plasmids in complex microbial communities. Specifically, we discuss how the emergence and persistence of MDR plasmids can be affected by varying selective conditions, spatial structure, environmental heterogeneity, temporal variation and coexistence with other members of the microbiome. These factors, along with others yet to be investigated, collectively determine the emergence and transfer of plasmid-mediated AMR within and between habitats at the local and global scale. In this Review, Castañeda-Barba, Top and Stalder use the One Health framework to synthesize the recent literature on the ecological and evolutionary factors that determine the successful local and global spread of plasmid-mediated antimicrobial resistance genes.
质粒,"一个健康 "时代抗菌药耐药性的分子基石。
抗菌药耐药性(AMR)对人类健康构成了巨大威胁。AMR的广泛流行部分是由于抗生素耐药性基因(ARG)的横向转移,通常由质粒介导。病原体中许多由质粒介导的抗性基因来自环境、动物或人类栖息地。尽管有证据表明质粒会在这些栖息地之间调动 ARGs,但我们对促进临床病原体出现耐多药(MDR)质粒的生态和进化轨迹了解有限。一个健康"(One Health)作为一个整体框架,可以帮助我们探索这些知识缺口。在本综述中,我们将概述质粒如何推动本地和全球 AMR 传播并将不同的栖息地联系起来。我们从生态进化的角度探讨了一些新出现的研究,并就影响质粒在复杂微生物群落中的生态和进化的因素展开了讨论。具体来说,我们将讨论 MDR 质粒的出现和持久性如何受到不同选择条件、空间结构、环境异质性、时间变化以及与微生物群其他成员共存的影响。这些因素以及其他有待研究的因素共同决定了质粒介导的 AMR 在本地和全球范围内的出现和转移。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Reviews Microbiology
生物-微生物学
CiteScore
74.00
自引率
0.50%
发文量
149
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Microbiology, our goal is to become the leading source of reviews and commentaries for the scientific community we cater to. We are dedicated to publishing articles that are not only authoritative but also easily accessible, supplementing them with clear and concise figures, tables, and other visual aids. Our objective is to offer an unparalleled service to authors, referees, and readers, and we continuously strive to maximize the usefulness and impact of each article we publish. With a focus on Reviews, Perspectives, and Comments spanning the entire field of microbiology, our wide scope ensures that the work we feature reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


