The Use of Biomarkers in Pharmacovigilance: A Systematic Review of the Literature.
IF 2.6
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
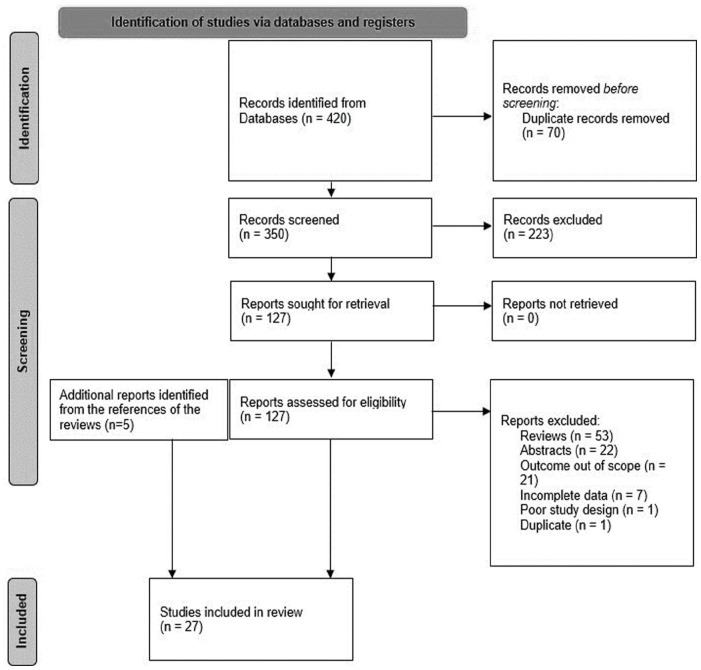
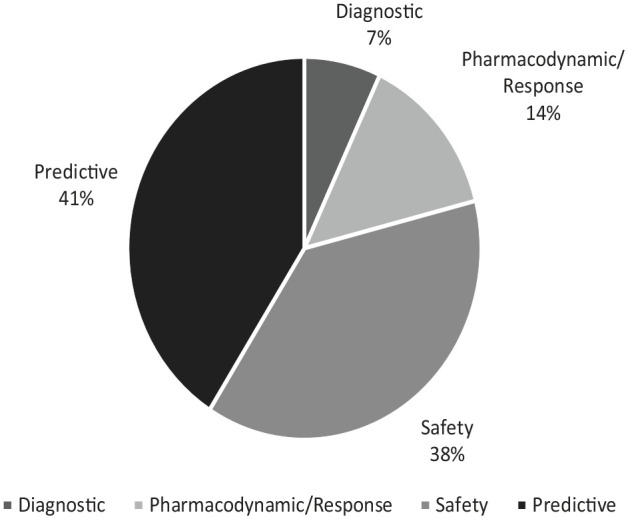
Background The use of biomarkers varies from disease etiognosis and diagnosis to signal detection, risk prediction, and management. Biomarker use has expanded in recent years, however, there are limited reviews on the use of biomarkers in pharmacovigilance and specifically in the monitoring and management of adverse drug reactions (ADRs). Objective The objective of this manuscript is to identify the multiple uses of biomarkers in pharmacovigilance irrespective of the therapeutic area. Design This is a systematic review of the literature. Data Sources and Methods Embase and MEDLINE database searches were conducted for literature published between 2010-March 19, 2021. Scientific articles that described the potential use of biomarkers in pharmacovigilance in sufficient detail were reviewed. Papers that did not fulfill the United States Food and Drug Administration (US FDA) definition of a biomarker were excluded, which is based on the International Conference on Harmonisation (ICH)-E16 guidance. Results Twenty-seven articles were identified for evaluation. Most articles involved predictive biomarkers (41%), followed by safety biomarkers (38%), pharmacodynamic/response biomarkers (14%), and diagnostic biomarkers (7%). Some articles described biomarkers that applied to multiple categories. Conclusion Various categories of biomarkers including safety, predictive, pharmacodynamic/response, and diagnostic biomarkers are being investigated for potential use in pharmacovigilance. The most frequent potential uses of biomarkers in pharmacovigilance in the literature were the prediction of the severity of an ADR, mortality, response, safety, and toxicity. The safety biomarkers identified were used to evaluate patient safety during dose escalation, identify patients who may benefit from further biomarker testing during treatment, and monitor ADRs.

生物标志物在药物警戒中的应用:文献系统综述。
背景:生物标志物的使用从疾病的病因诊断到信号检测、风险预测和管理各不相同。近年来,生物标志物的应用已经扩大,然而,关于生物标志物在药物警戒特别是药物不良反应(adr)监测和管理中的应用的综述有限。目的:本文的目的是确定生物标志物在药物警戒中的多种用途,而不考虑治疗领域。设计:这是一篇系统的文献综述。数据来源和方法:对2010年至2021年3月19日发表的文献进行Embase和MEDLINE数据库检索。对详细描述生物标志物在药物警戒中的潜在应用的科学文章进行了综述。不符合美国食品和药物管理局(FDA)生物标志物定义的论文被排除在外,这是基于国际协调会议(ICH)-E16指南。结果:筛选出27篇文献进行评价。大多数文章涉及预测性生物标志物(41%),其次是安全性生物标志物(38%),药效学/反应性生物标志物(14%)和诊断性生物标志物(7%)。一些文章描述了应用于多个类别的生物标志物。结论:各种生物标志物,包括安全性、预测性、药效学/反应性和诊断性生物标志物,正在研究其在药物警戒中的潜在用途。在文献中,生物标志物在药物警戒中最常见的潜在用途是预测不良反应的严重程度、死亡率、反应、安全性和毒性。确定的安全性生物标志物用于评估剂量递增期间患者的安全性,确定可能从治疗期间进一步生物标志物检测中受益的患者,并监测不良反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomarker Insights
MEDICINE, RESEARCH & EXPERIMENTAL-
CiteScore
6.00
自引率
0.00%
发文量
26
审稿时长
8 weeks
期刊介绍:
An open access, peer reviewed electronic journal that covers all aspects of biomarker research and clinical applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


