Real-world experience of monoclonal antibodies in mild-to-moderate COVID-19 patients at a tertiary care center
Q2 Medicine
引用次数: 0
Abstract
Background
Neutralizing antibodies cocktail (casirivimab and imdevimab) has received emergency use authorization recommendation by Food and Drug Administration (FDA) and WHO for mild-to-moderate COVID-19 infection in specific high-risk groups. Antibodies cocktail has shown promising results in preventing progression to severe disease, but the real-world experience is still evolving. Herein, we present a retrospective analysis of 22 patients who were administered the antibodies cocktail between August 2021 and March 2022 at our tertiary care center.
Methods
We conducted an observational retrospective analysis of clinicoradiological, inflammatory parameters, progression of the disease, and outcome among 22 mild and moderate COVID-19 patients treated with antibodies cocktail.
Results
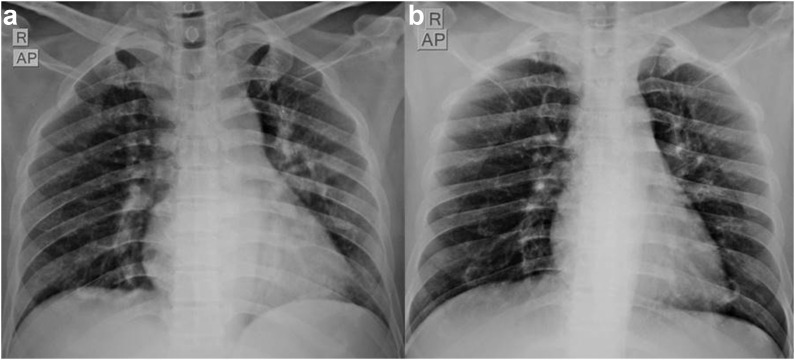
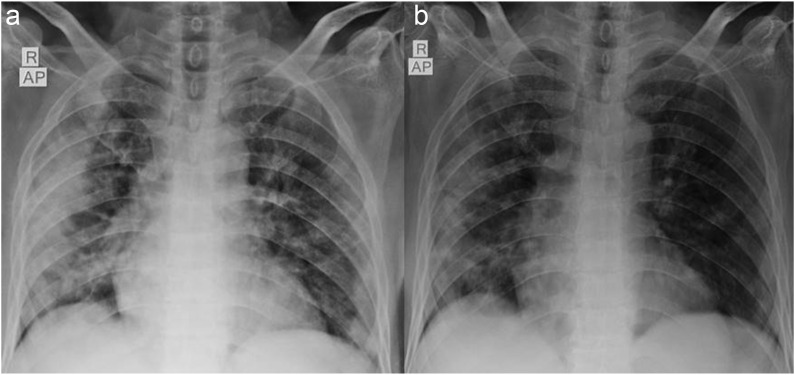
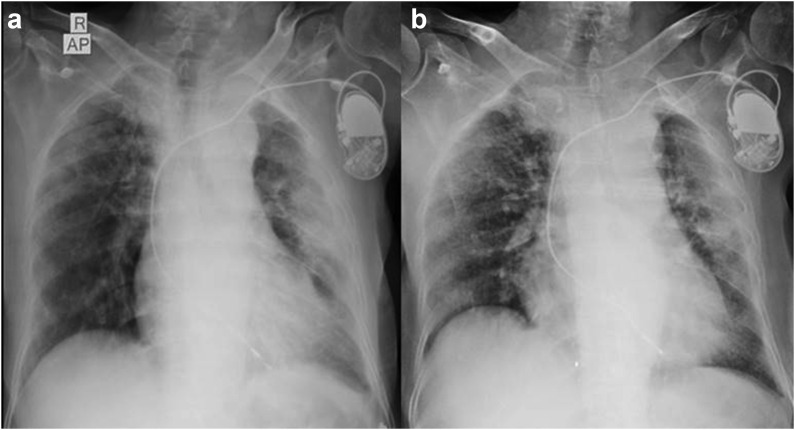
The mean age was 67.7 years (SD ± 18.3) and comprised of 13 males (59%), while 9 were females (40.9%). Nine (40.9%) patients were fully vaccinated with two doses, nine (40.9%) were partially vaccinated with one dose while four patients (18.2%) were unvaccinated, and the rest were unvaccinated. Diabetes and hypertension were the commonest comorbidities; hematological and solid organ malignancies were other comorbidities. Eight patients had radiological opacities consistent with COVID-19 pneumonia and had shown significant regression in four patients after the therapy. None of our patients required supplemental oxygen or progressed to severe acute respiratory distress syndrome. All patients were discharged in a stable condition within 6 days of the therapy.
Conclusions
The neutralizing antibodies cocktail has shown encouraging results in our analysis in preventing progression to severe disease in patients with high-risk conditions.
三级医疗中心轻至中度COVID-19患者单克隆抗体的实际经验
中和抗体鸡尾酒(卡西维单抗和伊德维单抗)已获得美国食品和药物管理局(FDA)和世卫组织的紧急使用授权建议,用于特定高危人群的轻中度COVID-19感染。抗体鸡尾酒在预防严重疾病进展方面显示出有希望的结果,但实际经验仍在不断发展。在此,我们对22名患者进行了回顾性分析,这些患者在2021年8月至2022年3月期间在我们的三级保健中心接受了抗体鸡尾酒治疗。方法观察回顾性分析22例接受抗体鸡尾酒治疗的轻中度COVID-19患者的临床放射学、炎症参数、疾病进展和结局。结果患者平均年龄67.7岁(SD±18.3),男性13例(59%),女性9例(40.9%)。2剂完全接种9例(40.9%),1剂部分接种9例(40.9%),未接种4例(18.2%),其余未接种。糖尿病和高血压是最常见的合并症;血液学和实体器官恶性肿瘤是其他合并症。8例患者出现符合COVID-19肺炎的影像学混浊,4例患者治疗后出现明显消退。没有患者需要补充氧气或进展为严重的急性呼吸窘迫综合征。所有患者均在治疗后6天出院,病情稳定。结论在我们的分析中,中和抗体鸡尾酒在预防高危患者发展为严重疾病方面显示出令人鼓舞的结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Medical Journal Armed Forces India
Medicine-Medicine (all)
CiteScore
3.40
自引率
0.00%
发文量
206
期刊介绍:
This journal was conceived in 1945 as the Journal of Indian Army Medical Corps. Col DR Thapar was the first Editor who published it on behalf of Lt. Gen Gordon Wilson, the then Director of Medical Services in India. Over the years the journal has achieved various milestones. Presently it is published in Vancouver style, printed on offset, and has a distribution exceeding 5000 per issue. It is published in January, April, July and October each year.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


