Genetic Data Governance in Japanese Hospitals
Abstract
Abstract
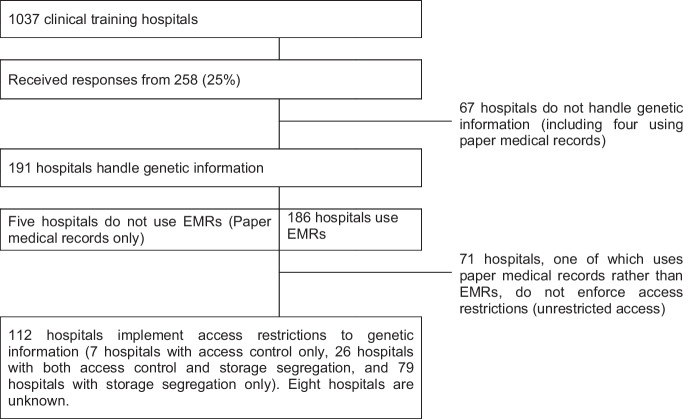
The storage and access of genetic testing results have unique considerations for medical records. Initially, genetic testing was limited to patients with single gene diseases. Genetic medicine and testing have expanded, as have concerns about appropriately handling genetic information. In this study, we surveyed the management of genetic information in general hospitals in Japan using a questionnaire on access restrictions. Our questions included whether any other medical information was managed in a unique way. We identified 1037 hospitals designated for clinical training located throughout Japan and received responses from 258 hospitals, and 191 reported that they handle genetic information and results of genetic tests. Of the 191 hospitals that handle genetic information, 112 hospitals implement access restrictions to genetic information. Seventy-one hospitals, one of which uses paper medical records rather than electrical medical records, do not enforce access restrictions. For eight hospitals, it was not known whether access restrictions were enforced or not. The responses from these hospitals indicated that access restrictions and storage methods varied across institution type (e.g., general vs. university hospitals), institution size, and the presence of a clinical genetics department. Other information, such as infectious disease diagnosis, psychological counseling records, abuse, and criminal history, was also subject to access restriction in 42 hospitals. The disparity in how medical facilities handle sensitive genetic information demonstrates a need for discussion between medical professionals and the general public on the storage of sensitive records, including genetic information.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


