Inaccuracies in plasma oxytocin extraction and enzyme immunoassay techniques
Abstract
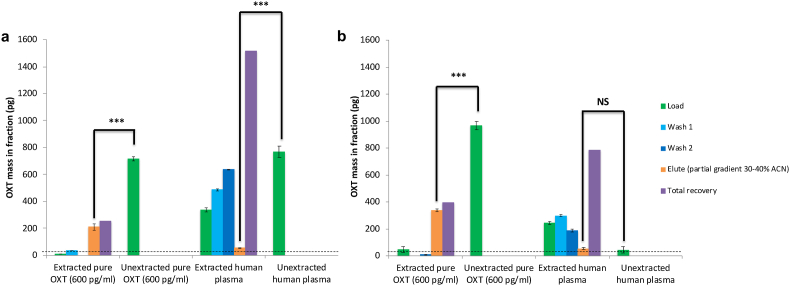
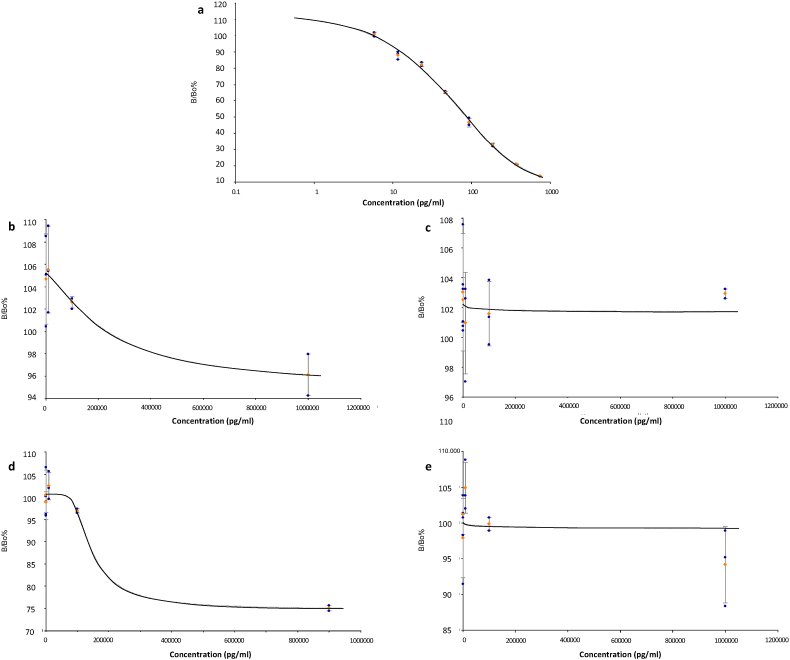
Numerous studies have reported extensive associations between plasma oxytocin (OXT) concentrations and various human physiological and neurobehavioral processes. Measurement of OXT is fraught with difficulty due to its low molecular weight and plasma concentrations, with no consensus as to the optimal conditions for pre-analytical sample extraction, standards for immunoassay validation or the ideal protease inhibitors to prevent OXT degradation. Previous attempts at determining the efficacy of various purification techniques such as solid phase extraction (SPE) or ultrafiltration have only utilized human plasma samples, making it difficult to dissect out whether the effect of interference comes from the extraction process itself or cross-reactivity with other proteins. By testing these on pure OXT solutions, we demonstrate poor recovery efficacy and reliability of reversed phase SPE (maximum 58.1%) and ultrafiltration (<1%) techniques, and the potential for the former to introduce interference into enzyme immunoassay (EIA) measurements. The clonality of antibodies used in EIA kits also potentially contributes to the differences in the readings obtained, and we validate an EIA kit which did not require pre-analytical sample extraction with low cross-reactivity and high reliability (intraclass correlation coefficient 0.980 (95% CI 0.896–0.999). Biochemical techniques used for measuring plasma OXT concentrations must therefore be internally validated prior to translation into clinical studies.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


