Neutrophil Counts in Healthy South African Infants: Implications for Enrollment and Adverse Event Grading in Clinical Trials in an African Setting
引用次数: 2
Abstract
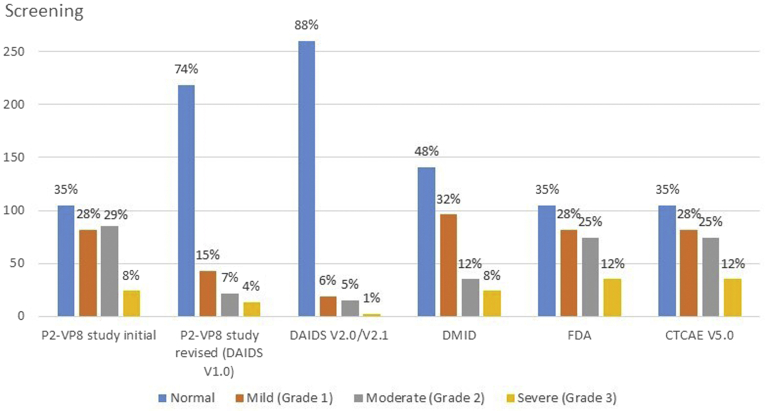
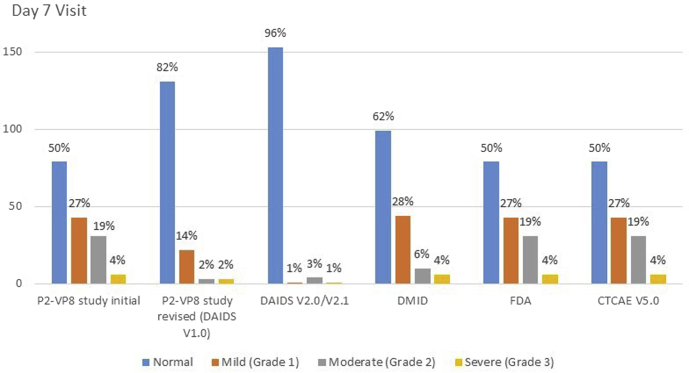
Absolute neutrophil counts are used to assess eligibility and safety during clinical trials but the toxicity grading scale used can affect enrollment and reporting of adverse events. During a trial investigating a parenteral rotavirus vaccine in South Africa, we excluded otherwise healthy infants without HIV infection from participation owing to neutropenia.
Trial registration
ClinicalTrials.gov: NCT02109484.
南非健康婴儿的中性粒细胞计数:在非洲环境下临床试验的入组和不良事件分级的含义
在临床试验中,绝对中性粒细胞计数用于评估合格性和安全性,但使用的毒性分级量表可能会影响不良事件的登记和报告。在南非一项调查肠外轮状病毒疫苗的试验中,由于中性粒细胞减少症,我们将未感染艾滋病毒的健康婴儿排除在外。临床试验注册:NCT02109484。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Pediatrics: X
Medicine-Pediatrics, Perinatology and Child Health
CiteScore
4.90
自引率
0.00%
发文量
0
审稿时长
23 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


