Inhibiting fatty acid synthesis overcomes colistin resistance
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 4
Abstract
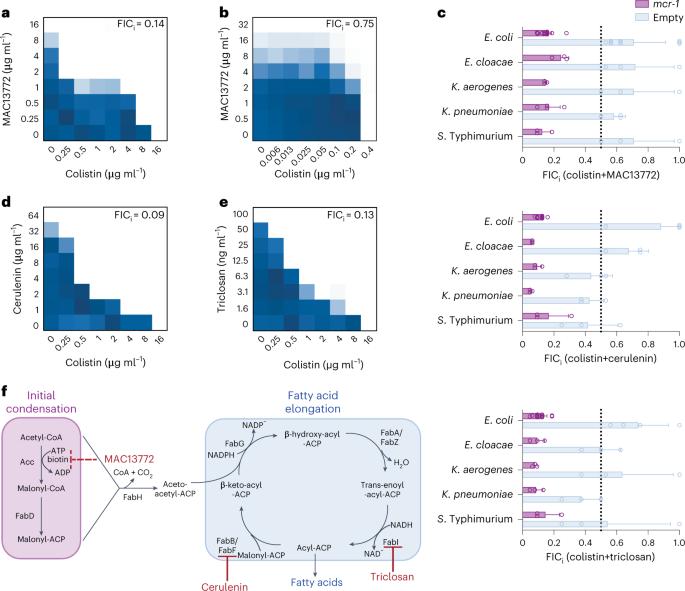
Treating multidrug-resistant infections has increasingly relied on last-resort antibiotics, including polymyxins, for example colistin. As polymyxins are given routinely, the prevalence of their resistance is on the rise and increases mortality rates of sepsis patients. The global dissemination of plasmid-borne colistin resistance, driven by the emergence of mcr-1, threatens to diminish the therapeutic utility of polymyxins from an already shrinking antibiotic arsenal. Restoring sensitivity to polymyxins using combination therapy with sensitizing drugs is a promising approach to reviving its clinical utility. Here we describe the ability of the biotin biosynthesis inhibitor, MAC13772, to synergize with colistin exclusively against colistin-resistant bacteria. MAC13772 indirectly disrupts fatty acid synthesis (FAS) and restores sensitivity to the last-resort antibiotic, colistin. Accordingly, we found that combinations of colistin and other FAS inhibitors, cerulenin, triclosan and Debio1452-NH3, had broad potential against both chromosomal and plasmid-mediated colistin resistance in chequerboard and lysis assays. Furthermore, combination therapy with colistin and the clinically relevant FabI inhibitor, Debio1452-NH3, showed efficacy against mcr-1 positive Klebsiella pneumoniae and colistin-resistant Escherichia coli systemic infections in mice. Using chemical genomics, lipidomics and transcriptomics, we explored the mechanism of the interaction. We propose that inhibiting FAS restores colistin sensitivity by depleting lipid synthesis, leading to changes in phospholipid composition. In all, this work reveals a surprising link between FAS and colistin resistance. Inhibition of fatty acid biosynthesis re-sensitizes colistin-resistant clinically relevant bacteria in vivo by inducing stress responses and altering membrane composition.
抑制脂肪酸合成可克服秋水仙碱耐药性
治疗耐多药感染越来越多地依赖于最后的抗生素,包括多粘菌素,例如可乐定。随着多粘菌素的常规使用,其耐药性也在不断上升,并增加了败血症患者的死亡率。在 mcr-1 出现的推动下,质粒携带的大肠菌素耐药性在全球范围内蔓延,这有可能削弱多粘菌素在本已日渐萎缩的抗生素库中的治疗作用。通过与增敏药物联合治疗来恢复多粘菌素的敏感性,是恢复多粘菌素临床用途的一种很有前景的方法。在这里,我们描述了生物素生物合成抑制剂 MAC13772 与秋水仙素协同作用专门对付耐秋水仙素细菌的能力。MAC13772 间接破坏了脂肪酸合成(FAS),并恢复了对最后一种抗生素秋水仙素的敏感性。因此,我们发现,在棋盘试验和裂解试验中,秋水仙素与其他脂肪酸抑制剂(cerulenin、三氯生和 Debio1452-NH3)的组合具有广泛的潜力,可对抗染色体和质粒介导的秋水仙素耐药性。此外,使用秋水仙素和与临床相关的 FabI 抑制剂 Debio1452-NH3 进行联合治疗,对 mcr-1 阳性肺炎克雷伯菌和耐秋水仙素大肠埃希菌的小鼠全身感染具有疗效。我们利用化学基因组学、脂质组学和转录组学探索了这种相互作用的机制。我们提出,抑制 FAS 会消耗脂质合成,导致磷脂组成发生变化,从而恢复对可乐定的敏感性。总之,这项工作揭示了 FAS 与可乐定耐药性之间的惊人联系。通过诱导应激反应和改变膜组成,抑制脂肪酸的生物合成可使体内对临床相关的可乐定耐药细菌重新敏感。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


