A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 35
Abstract
The ionic conductivity of composite solid-state electrolytes does not meet the application requirements of solid-state lithium (Li) metal batteries owing to the harsh space charge layer of different phases and low concentration of movable Li+. Herein, we propose a robust strategy for creating high-throughput Li+ transport pathways by coupling the ceramic dielectric and electrolyte to overcome the low ionic conductivity challenge of composite solid-state electrolytes. A highly conductive and dielectric composite solid-state electrolyte is constructed by compositing the poly(vinylidene difluoride) matrix and the BaTiO3–Li0.33La0.56TiO3–x nanowires with a side-by-side heterojunction structure (PVBL). The polarized dielectric BaTiO3 greatly promotes the dissociation of Li salt to produce more movable Li+, which locally and spontaneously transfers across the interface to coupled Li0.33La0.56TiO3–x for highly efficient transport. The BaTiO3–Li0.33La0.56TiO3–x effectively restrains the formation of the space charge layer with poly(vinylidene difluoride). These coupling effects contribute to a quite high ionic conductivity (8.2 × 10−4 S cm−1) and lithium transference number (0.57) of the PVBL at 25 °C. The PVBL also homogenizes the interfacial electric field with electrodes. The LiNi0.8Co0.1Mn0.1O2/PVBL/Li solid-state batteries stably cycle 1,500 times at a current density of 180 mA g−1, and pouch batteries also exhibit an excellent electrochemical and safety performance. The authors developed a highly conductive and dielectric composite solid-state electrolyte by coupling BaTiO3 and Li0.33La0.56TiO3–x nanowires with a side-by-side heterojunction structure in a polyvinylidene difluoride matrix, which simultaneously promotes the dissociation of lithium salts to produce more movable Li ions and efficiently transports the generated movable Li ions.
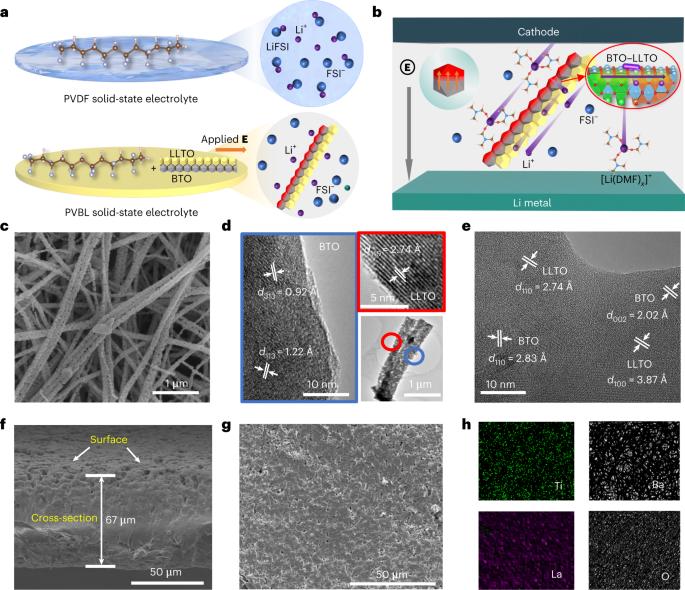
用于高压固态锂金属电池的高锂离子传导性电介质复合材料
复合固态电解质的离子电导率不符合固态锂(Li)金属电池的应用要求,原因是不同相的空间电荷层非常苛刻,且可移动 Li+ 的浓度较低。在此,我们提出了一种稳健的策略,通过耦合陶瓷电介质和电解质来创建高通量的 Li+ 传输通道,从而克服复合固态电解质离子电导率低的难题。通过将聚(偏二氟乙烯)基质和具有并排异质结结构(PVBL)的 BaTiO3-Li0.33La0.56TiO3-x 纳米线复合在一起,构建了一种高导电性和介电性的复合固态电解质。极化电介质 BaTiO3 能极大地促进锂盐的解离,从而产生更多可移动的 Li+,这些 Li+能局部自发地穿过界面转移到耦合的 Li0.33La0.56TiO3-x 上,从而实现高效传输。BaTiO3-Li0.33La0.56TiO3-x 能有效抑制聚偏二氟乙烯空间电荷层的形成。这些耦合效应使得 PVBL 在 25 °C 时具有相当高的离子电导率(8.2 × 10-4 S cm-1)和锂转移数(0.57)。PVBL 还能均匀化与电极之间的界面电场。锂镍0.8Co0.1Mn0.1O2/PVBL/锂固态电池在电流密度为180 mA g-1的条件下可稳定循环1500次,而且袋装电池还表现出优异的电化学和安全性能。作者通过在聚偏二氟乙烯基质中耦合具有并排异质结结构的 BaTiO3 和 Li0.33La0.56TiO3-x 纳米线,开发出了一种高导电性和介电性复合固态电解质,它能同时促进锂盐的解离以产生更多的可移动锂离子,并高效地传输所产生的可移动锂离子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


