On the Redox Properties of the Dimers of Thiazol-2-ylidenes That Are Relevant for Radical Catalysis
IF 3.3
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
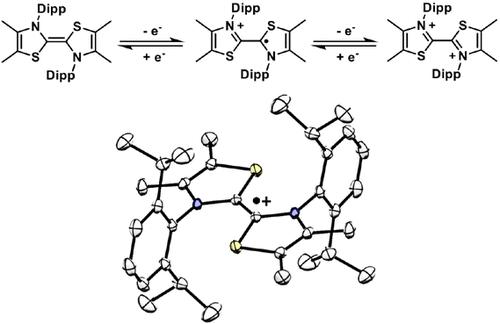
We report the isolation and study of dimers stemming from popular thiazol-2-ylidene organocatalysts. The model featuring 2,6-di(isopropyl)phenyl (Dipp) N-substituents was found to be a stronger reducing agent (Eox = −0.8 V vs SCE) than bis(thiazol-2-ylidenes) previously studied in the literature. In addition, a remarkable potential gap between the first and second oxidation of the dimer also allows for the isolation of the corresponding air-persistent radical cation. The latter is an unexpected efficient promoter of the radical transformation of α-bromoamides into oxindoles.
与自由基催化有关的噻唑-2-吡啶二聚体的氧化还原性质研究
我们报道了从流行的噻唑-2-亚基有机催化剂中分离和研究二聚体。以2,6-二(异丙基)苯基(Dip)N-取代基为特征的模型被发现是比文献中先前研究的双(噻唑-2-亚基)更强的还原剂(Eox=−0.8 V vs SCE)。此外,二聚体的第一次和第二次氧化之间的显著电势差也允许分离相应的空气持久性自由基阳离子。后者是α-溴酰胺自由基转化为羟吲哚的出乎意料的有效促进剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Organic & Inorganic Au
有机化学、无机化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Organic & Inorganic Au is an open access journal that publishes original experimental and theoretical/computational studies on organic organometallic inorganic crystal growth and engineering and organic process chemistry. Short letters comprehensive articles reviews and perspectives are welcome on topics that include:Organic chemistry Organometallic chemistry Inorganic Chemistry and Organic Process Chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


