The Clinical Efficacy, Safety, and Tolerability of Vancomycin for the Treatment of Recurrent Clostridioides difficile Infection - A Systematic Review.
IF 3.4
Q2 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
Abstract
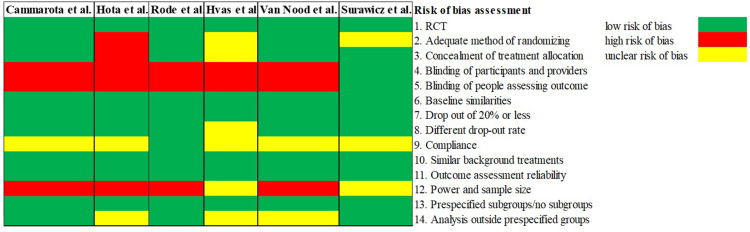
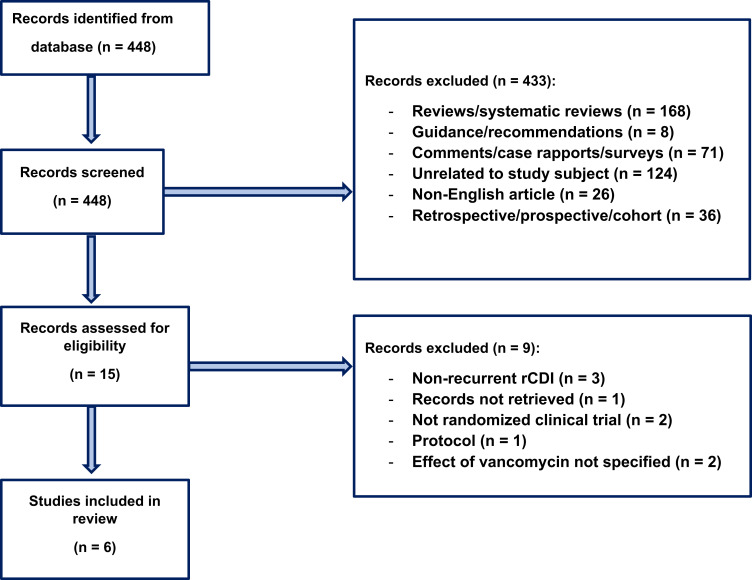
Introduction The aim of this systematic review of randomized clinical trials (RCTs) was to examine the efficacy, safety, and tolerability of vancomycin for treatment of recurrent Clostridioides difficile infection (rCDI). Methods The PubMed database was searched from inception to August 23, 2022. An initial screening was performed followed by a full-text evaluation of the papers. Inclusion criteria were RCTs investigating vancomycin for treatment of rCDI. Results A total of six studies and 269 patients were included in the review. Three studies used a fixed dose regimen of vancomycin, one study used pulse regimen, one study used a taper-and-pulse regimen, and one study used a taper-and-pulse regimen for the participants with two or more recurrences. The resolution of infection varied from 19% to 58.3% in five of six studies reporting this as an outcome. Four out of six studies reported new episodes of rCDI as an intervention outcome, in those studies 50–63% of participants experienced rCDI. Regarding the safety and tolerability of vancomycin treatment for rCDI, one study described several adverse events regarding gastrointestinal discomfort along with fatigue and skin rash. There were no records of serious adverse events in the included studies. Conclusion While oral vancomycin is mostly safe and well tolerated in the RCTs reviewed here, the efficacy for treating rCDI varies greatly from 19–58.3%, and 50–63% of participants experienced new episodes of rCDI.
万古霉素治疗复发性艰难梭菌感染的临床疗效、安全性和耐受性——系统综述。
简介:本系统综述随机临床试验(RCTs)的目的是研究万古霉素治疗复发性艰难梭菌感染(rCDI)的有效性、安全性和耐受性。方法:检索PubMed数据库自成立至2022年8月23日。首先进行初步筛选,然后对论文进行全文评估。纳入标准为研究万古霉素治疗rCDI的随机对照试验。结果:共纳入6项研究和269例患者。三项研究使用固定剂量万古霉素治疗方案,一项研究使用脉冲治疗方案,一项研究使用锥形脉冲治疗方案,一项研究使用锥形脉冲治疗方案,一项研究对两次或两次以上复发的参与者使用锥形脉冲治疗方案。在报道这一结果的6项研究中,有5项研究的感染率从19%到58.3%不等。6项研究中有4项报告了rCDI的新发作作为干预结果,在这些研究中,50-63%的参与者经历了rCDI。关于万古霉素治疗rCDI的安全性和耐受性,一项研究描述了胃肠道不适、疲劳和皮疹等不良事件。在纳入的研究中没有严重不良事件的记录。结论:虽然在本文回顾的随机对照试验中,口服万古霉素大多是安全且耐受性良好的,但治疗rCDI的疗效差异很大,在19-58.3%之间,50-63%的参与者出现了新的rCDI发作。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Drug, Healthcare and Patient Safety
HEALTH CARE SCIENCES & SERVICES-
CiteScore
4.10
自引率
0.00%
发文量
24
审稿时长
16 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


