Magnetic Microrobots with Folate Targeting for Drug Delivery.
IF 18.1
Q1 ENGINEERING, BIOMEDICAL
引用次数: 8
Abstract
Untethered microrobots can be used for cargo delivery (e.g., drug molecules, stem cells, and genes) targeting designated areas. However, it is not enough to just reach the lesion site, as some drugs can only play the best therapeutic effect within the cells. To this end, folic acid (FA) was introduced into microrobots in this work as a key to mediate endocytosis of drugs into cells. The microrobots here were fabricated with biodegradable gelatin methacryloyl (GelMA) and modified with magnetic metal–organic framework (MOF). The porous structure of MOF and the hydrogel network of polymerized GelMA were used for the loading of enough FA and anticancer drug doxorubicin (DOX) respectively. Utilizing the magnetic property of magnetic MOF, these microrobots can gather around the lesion site with the navigation of magnetic fields. The combination effects of FA targeting and magnetic navigation substantially improve the anticancer efficiency of these microrobots. The result shows that the cancer cells inhibition rate of microrobots with FA can be up to 93%, while that of the ones without FA was only 78%. The introduction of FA is a useful method to improve the drug transportation ability of microrobots, providing a meaningful reference for further research.
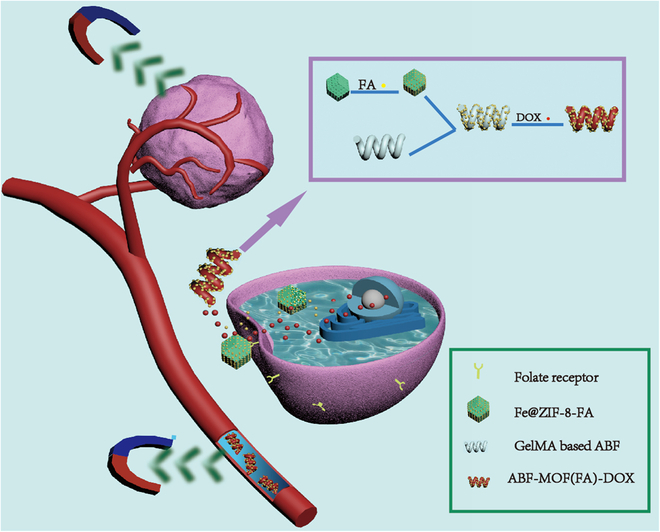
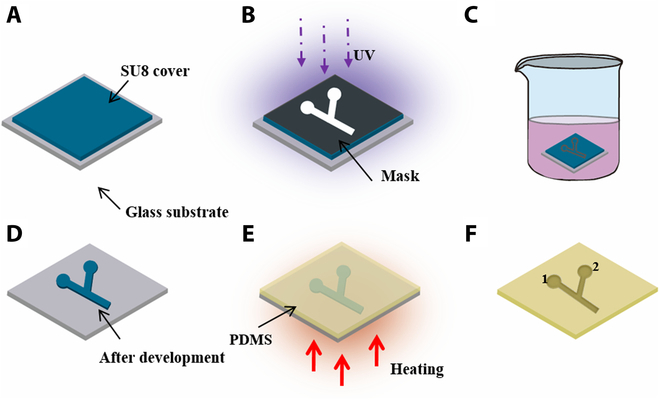
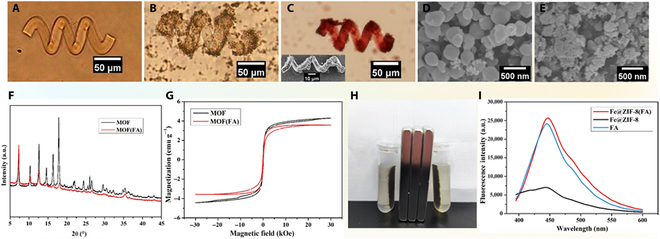
具有叶酸靶向药物递送的磁性微型机器人。
不受束缚的微型机器人可用于针对指定区域的货物运输(例如,药物分子、干细胞和基因)。然而,仅仅到达病变部位是不够的,因为有些药物只能在细胞内发挥最佳的治疗效果。为此,本研究将叶酸(folic acid, FA)作为介导药物内吞作用的关键物质引入微机器人。该微机器人由可生物降解的明胶甲基丙烯酰(GelMA)和磁性金属有机框架(MOF)修饰而成。利用MOF的多孔结构和聚合GelMA的水凝胶网络分别装载足量FA和抗癌药物DOX。利用磁性MOF的磁性,这些微型机器人可以在磁场的导航下聚集在病变部位周围。FA靶向和磁导航的联合作用大大提高了这些微型机器人的抗癌效率。结果表明,添加FA的微型机器人对癌细胞的抑制率可达93%,而不添加FA的微型机器人的抑制率仅为78%。FA的引入为提高微型机器人的药物运输能力提供了有益的方法,为进一步的研究提供了有意义的参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


