Liver tumour immune microenvironment subtypes and neutrophil heterogeneity
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 89
Abstract
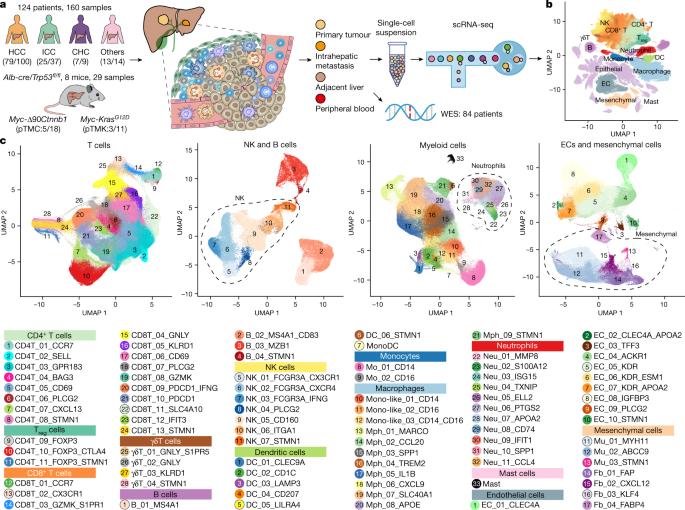
The heterogeneity of the tumour immune microenvironment (TIME), organized by various immune and stromal cells, is a major contributing factor of tumour metastasis, relapse and drug resistance1–3, but how different TIME subtypes are connected to the clinical relevance in liver cancer remains unclear. Here we performed single-cell RNA-sequencing (scRNA-seq) analysis of 189 samples collected from 124 patients and 8 mice with liver cancer. With more than 1 million cells analysed, we stratified patients into five TIME subtypes, including immune activation, immune suppression mediated by myeloid or stromal cells, immune exclusion and immune residence phenotypes. Different TIME subtypes were spatially organized and associated with chemokine networks and genomic features. Notably, tumour-associated neutrophil (TAN) populations enriched in the myeloid-cell-enriched subtype were associated with an unfavourable prognosis. Through in vitro induction of TANs and ex vivo analyses of patient TANs, we showed that CCL4+ TANs can recruit macrophages and that PD-L1+ TANs can suppress T cell cytotoxicity. Furthermore, scRNA-seq analysis of mouse neutrophil subsets revealed that they are largely conserved with those of humans. In vivo neutrophil depletion in mouse models attenuated tumour progression, confirming the pro-tumour phenotypes of TANs. With this detailed cellular heterogeneity landscape of liver cancer, our study illustrates diverse TIME subtypes, highlights immunosuppressive functions of TANs and sheds light on potential immunotherapies targeting TANs. Tumour-associated neutrophil populations enriched in the myeloid-cell-enriched tumour immune microenvironment subtype are associated with unfavourable prognosis in humans and mice with liver cancer.
肝脏肿瘤免疫微环境亚型和中性粒细胞异质性
由各种免疫细胞和基质细胞组织的肿瘤免疫微环境(TIME)的异质性是导致肿瘤转移、复发和耐药性的主要因素1-3,但不同的TIME亚型如何与肝癌的临床相关性联系在一起仍不清楚。在这里,我们对从124名肝癌患者和8只小鼠身上采集的189个样本进行了单细胞RNA测序(scRNA-seq)分析。通过对超过 100 万个细胞的分析,我们将患者分为五种 TIME 亚型,包括免疫激活、髓样细胞或基质细胞介导的免疫抑制、免疫排斥和免疫驻留表型。不同的TIME亚型在空间上是有组织的,并与趋化因子网络和基因组特征相关。值得注意的是,富含髓系细胞亚型的肿瘤相关中性粒细胞(TAN)群体与不良预后有关。通过体外诱导 TANs 和患者 TANs 的体外分析,我们发现 CCL4+ TANs 可以招募巨噬细胞,PD-L1+ TANs 可以抑制 T 细胞的细胞毒性。此外,对小鼠中性粒细胞亚群的 scRNA-seq 分析表明,它们在很大程度上与人类中性粒细胞亚群保持一致。在小鼠模型中体内中性粒细胞耗竭可减轻肿瘤进展,这证实了TANs的促肿瘤表型。通过这一肝癌细胞异质性的详细图谱,我们的研究说明了肝癌的多种亚型,强调了TANs的免疫抑制功能,并揭示了针对TANs的潜在免疫疗法。富含髓系细胞的肿瘤免疫微环境亚型中的肿瘤相关中性粒细胞群与人类和小鼠肝癌患者的不良预后有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


