Tranilast-matrine co-amorphous system: Strong intermolecular interactions, improved solubility, and physiochemical stability
Abstract
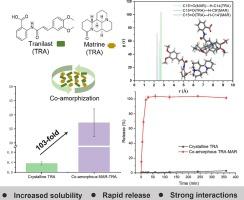
There is a great interest to develop co-amorphous drug delivery systems to enhance the solubility of biopharmaceutics classification system (BCS) class II and IV drugs. However, most reported systems only resulted in severalfold solubility improvement. Tranilast (TRA) is an anti-allergic drug used to treat bronchial asthma and allergic rhinitis. It is a BCS class II drug and its poor aqueous solubility affects its absorption in vivo. To address this issue, a natural alkaloid matrine (MAR) with interesting biological activities was chosen to form a co-amorphous system with TRA, based on the solubility parameter and phase solubility experiment. The TRA-MAR drug-drug co-amorphous system was prepared by the solvent evaporation method, and further characterized by powder X-ray diffraction and modulated temperature differential scanning calorimetry. Fourier transform infrared spectroscopy, FT-Raman, and X-ray photoelectron spectroscopy revealed the formation of salt and the presence of strong intermolecular interactions in the TRA-MAR co-amorphous system, which are also supported by molecular dynamics simulations, showing ionic and hydrogen bonding interactions. This co-amorphous system exhibited excellent physical stability at both 25 °C and 40 °C under anhydrous silica gel condition. Finally, co-amorphous TRA-MAR showed greatly enhanced solubility (greater than 100-fold) and rapid release behavior in the vitro release experiments. NMR spectroscopy revealed the strong intermolecular interactions between TRA and MAR in both DMSO‑d6 and D2O. Our study resulted in a TRA-MAR co-amorphous drug system with significant solubility improvement and showcased the great potential to improve the dissolution behaviors of BCS class II and IV drugs through the co-amorphization approach.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


