TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 6
Abstract
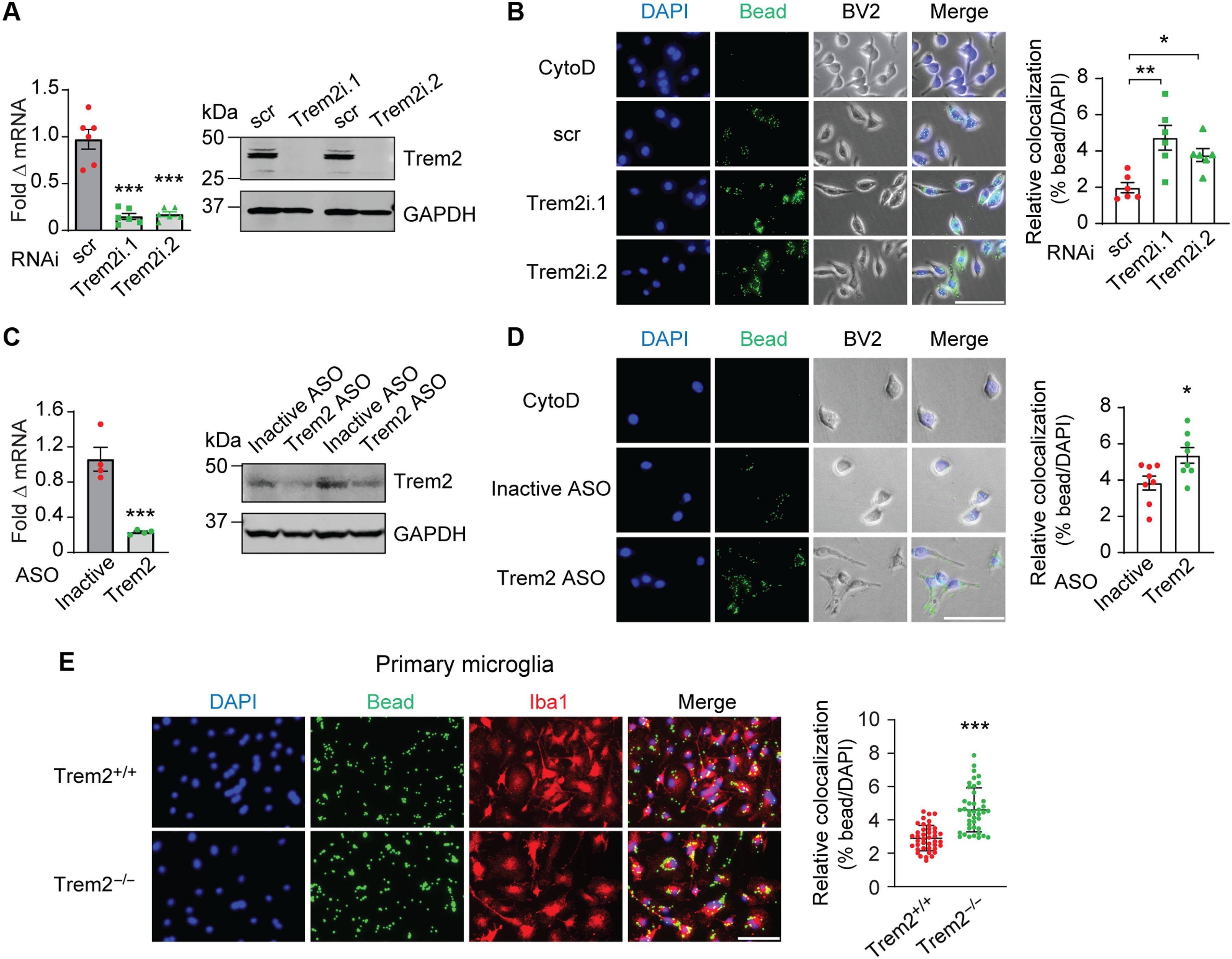
Triggering receptor expressed on myeloid cells 2 (TREM2) plays important roles in brain microglial function in neurodegenerative diseases, but the role of TREM2 in the GBM TME has not been examined. Here, we found that TREM2 is highly expressed in myeloid subsets, including macrophages and microglia in human and mouse GBM tumors and that high TREM2 expression correlates with poor prognosis in patients with GBM. TREM2 loss of function in human macrophages and mouse myeloid cells increased interferon-γ–induced immunoactivation, proinflammatory polarization, and tumoricidal capacity. In orthotopic mouse GBM models, mice with chronic and acute Trem2 loss of function exhibited decreased tumor growth and increased survival. Trem2 inhibition reprogrammed myeloid phenotypes and increased programmed cell death protein 1 (PD-1)+CD8+ T cells in the TME. Last, Trem2 deficiency enhanced the effectiveness of anti–PD-1 treatment, which may represent a therapeutic strategy for patients with GBM.
抑制 TREM2 可激发胶质母细胞瘤髓细胞的抗肿瘤细胞活性
髓系细胞上表达的触发受体2(TREM2)在神经退行性疾病的脑小胶质细胞功能中发挥着重要作用,但TREM2在GBM TME中的作用尚未得到研究。在这里,我们发现 TREM2 在人和小鼠 GBM 肿瘤的髓细胞亚群(包括巨噬细胞和小胶质细胞)中高表达,而且 TREM2 的高表达与 GBM 患者的不良预后相关。人巨噬细胞和小鼠髓系细胞中 TREM2 功能缺失会增加干扰素-γ 诱导的免疫激活、促炎极化和杀瘤能力。在正位小鼠脑胶质瘤模型中,慢性和急性 Trem2 功能丧失小鼠的肿瘤生长速度降低,存活率提高。抑制Trem2可重塑髓系表型,增加TME中的程序性细胞死亡蛋白1(PD-1)+CD8+ T细胞。最后,Trem2的缺失增强了抗PD-1治疗的效果,这可能是GBM患者的一种治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


