Lysine 2-hydroxyisobutyrylation of NAT10 promotes cancer metastasis in an ac4C-dependent manner
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 8
Abstract
Posttranslational modifications add tremendous complexity to proteomes; however, gaps remain in knowledge regarding the function and regulatory mechanism of newly discovered lysine acylation modifications. Here, we compared a panel of non-histone lysine acylation patterns in metastasis models and clinical samples, and focused on 2-hydroxyisobutyrylation (Khib) due to its significant upregulation in cancer metastases. By the integration of systemic Khib proteome profiling in 20 paired primary esophageal tumor and metastatic tumor tissues with CRISPR/Cas9 functional screening, we identified N-acetyltransferase 10 (NAT10) as a substrate for Khib modification. We further showed that Khib modification at lysine 823 in NAT10 functionally contribute to metastasis. Mechanistically, NAT10 Khib modification enhances its interaction with deubiquitinase USP39, resulting in increased NAT10 protein stability. NAT10 in turn promotes metastasis by increasing NOTCH3 mRNA stability in an N4-acetylcytidine-dependent manner. Furthermore, we discovered a lead compound #7586-3507 that inhibited NAT10 Khib modification and showed efficacy in tumor models in vivo at a low concentration. Together, our findings bridge newly identified lysine acylation modifications with RNA modifications, thus providing novel insights into epigenetic regulation in human cancer. We propose that pharmacological inhibition of NAT10 K823 Khib modification constitutes a potential anti-metastasis strategy.
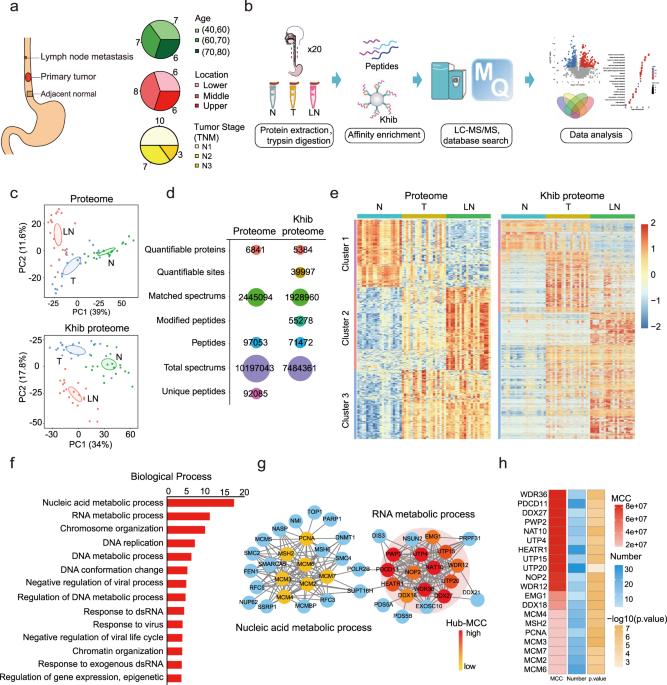
NAT10 的赖氨酸 2-羟基异丁酰化以依赖于 ac4C 的方式促进癌症转移
翻译后修饰增加了蛋白质组的巨大复杂性;然而,关于新发现的赖氨酸酰化修饰的功能和调控机制的知识仍然存在空白。在这里,我们比较了转移模型和临床样本中的一系列非组蛋白赖氨酸酰化模式,并重点研究了2-羟基异丁酰化(Khib),因为它在癌症转移中显著上调。通过对20个配对的原发性食管肿瘤和转移性肿瘤组织进行系统的Khib蛋白质组分析,并结合CRISPR/Cas9功能筛选,我们发现N-乙酰转移酶10(NAT10)是Khib修饰的底物。我们进一步发现,NAT10赖氨酸823处的Khib修饰在功能上有助于肿瘤转移。从机理上讲,NAT10的Khib修饰增强了它与去泛素化酶USP39的相互作用,从而提高了NAT10蛋白的稳定性。反过来,NAT10 又以 N4-乙酰胞嘧啶依赖的方式增加 NOTCH3 mRNA 的稳定性,从而促进转移。此外,我们还发现了一种先导化合物 #7586-3507,它能抑制 NAT10 Khib 修饰,并在体内肿瘤模型中显示出低浓度的疗效。总之,我们的发现将新发现的赖氨酸酰化修饰与 RNA 修饰联系在一起,从而为人类癌症的表观遗传调控提供了新的见解。我们认为药理抑制 NAT10 K823 Khib 修饰是一种潜在的抗肿瘤策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


