The differentiation and generation of glucose-sensitive beta like-cells from menstrual blood-derived stem cells using an optimized differentiation medium with platelet-rich plasma (PRP)
Abstract
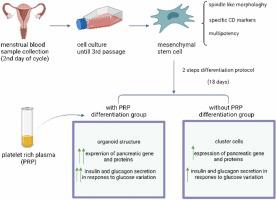
Regarding their reversible damage of insulin-producing cells (IPCs) and the inefficiency of treatment methods for type 1 diabetes mellitus (T1DM), scientists decided to produce IPCs from an unlimited source of cells. But the production of these cells is constantly faced with problems such as low differentiation efficiency in cell therapy and regenerative medicine. This study provided an ideal differentiation medium enriched with plasma-rich platelet (PRP) delivery to produce IPCs from menstrual blood-derived stem cells (MenSCs). We compared them with and without PRP differentiation medium. MenSCs were then cultured in two experimental groups: with/without PRP differentiation medium and a control group (undifferentiated MenSCs). After 18 days, differentiated cells were analyzed for expression of pancreatic gene markers by real-time PCR. Immunocytochemical staining was used to detect the presence of insulin and Pdx-1 in the differentiated cells, and insulin and C-peptide secretion response to glucose were tested by ELISA. Finally, the morphology of differentiated cells was examined by an inverted microscope. In vitro studies showed that MenSCs differentiated in the PRP differentiation medium had strong properties of IPCs such as pancreatic islet-like structure. The expression of pancreatic markers at both RNA and protein levels showed that the differentiation efficiency was higher in the PRP differentiation medium. In both experimental groups, the differentiated cells were functional and secreted C-peptide and insulin on glucose stimulation, but the secretion of C-peptide and insulin in the PRP group was higher than those cultured in the without PRP differentiation medium. Our findings showed that using of PRP enriched differentiation medium can promote the differentiation of MenSCs into IPCs compared to the without PRP culture group. Therefore, the use of PRP into differentiation media can be proposed as a new approach to producing IPCs from MenSCs and used in cell-based therapies for T1DM.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


