YcaO-mediated ATP-dependent peptidase activity in ribosomal peptide biosynthesis
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 4
Abstract
YcaO enzymes catalyze ATP-dependent post-translation modifications on peptides, including the installation of (ox/thi)azoline, thioamide and/or amidine moieties. Here we demonstrate that, in the biosynthesis of the bis-methyloxazolic alkaloid muscoride A, the YcaO enzyme MusD carries out both ATP-dependent cyclodehydration and peptide bond cleavage, which is a mechanism unprecedented for such a reaction. YcaO-catalyzed modifications are proposed to occur through a backbone O-phosphorylated intermediate, but this mechanism remains speculative. We report, to our knowedge, the first characterization of an acyl-phosphate species consistent with the proposed mechanism for backbone amide activation. The 3.1-Å-resolution cryogenic electron microscopy structure of MusD along with biochemical analysis allow identification of residues that enable peptide cleavage reaction. Bioinformatics analysis identifies other cyanobactin pathways that may deploy bifunctional YcaO enzymes. Our structural, mutational and mechanistic studies expand the scope of modifications catalyzed by YcaO proteins to include peptide hydrolysis and provide evidence for a unifying mechanism for the catalytically diverse outcomes. YcaO enzymes carry out diverse tailoring reactions of peptide-derived natural products, such as formation of rings and incorporation of sulfur, but YcaO enzymes also catalyze peptide proteolysis using adenosine 5′-triphosphosphate as a co-factor.
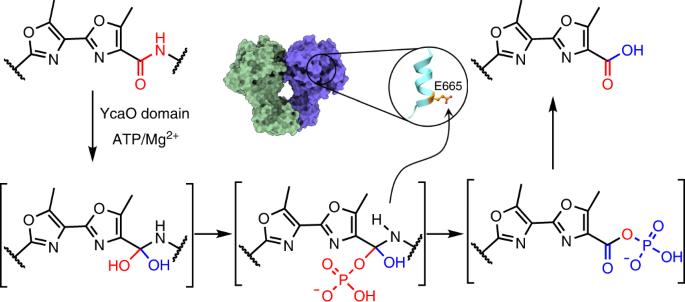
核糖体多肽生物合成中 YcaO 介导的 ATP 依赖性肽酶活性
YcaO 酶催化多肽翻译后的 ATP 依赖性修饰,包括安装(氧/硫)偶氮啉、硫酰胺和/或脒分子。在这里,我们证明了在双甲基恶唑生物碱麝香草内酯 A 的生物合成过程中,YcaO 酶 MusD 同时进行 ATP 依赖性环脱水和肽键裂解,这是此类反应前所未有的机制。有人提出,YcaO 催化的修饰是通过骨架 O-磷酸化中间体发生的,但这一机制仍然是推测性的。据我们所知,我们首次报告了与所提出的骨干酰胺活化机制相一致的酰基磷酸物种的特征。通过 3.1 Å 分辨率的 MusD 低温电子显微镜结构和生化分析,我们确定了能使肽裂解反应发生的残基。生物信息学分析确定了其他可能使用双功能 YcaO 酶的蓝藻毒素途径。我们的结构、突变和机理研究扩大了 YcaO 蛋白催化的修饰范围,包括肽水解,并为催化不同结果的统一机制提供了证据。YcaO酶能对肽类天然产物进行多种修饰反应,如形成环和加入硫,但YcaO酶还能利用5′-三磷酸腺苷作为辅助因子催化肽蛋白水解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


