A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 16
Abstract
C(sp3)-rich bicyclic hydrocarbon scaffolds, as exemplified by bicyclo[1.1.1]pentanes, play an increasingly high-profile role as saturated bioisosteres of benzenoids in medicinal chemistry and crop science. Substituted bicyclo[2.1.1]hexanes (BCHs) are emerging bicyclic hydrocarbon bioisosteres for ortho- and meta-substituted benzenes, but are difficult to access. Therefore, a general synthetic route to BCHs is needed if their potential as bioisosteres is to be realized. Here we describe a broadly applicable catalytic approach that delivers substituted BCHs by intermolecular coupling between olefins and bicyclo[1.1.0]butyl (BCB) ketones. The SmI2–catalysed process works for a wide range of electron-deficient alkenes and substituted BCB ketones, operates with SmI2 loadings as low as 5 mol% and is underpinned by a radical relay mechanism that is supported by density functional theory calculations. The product BCH ketones have been shown to be versatile synthetic intermediates through selective downstream manipulation and the expedient synthesis of a saturated hydrocarbon analogue of the broad-spectrum antimicrobial, phthalylsulfathiazole. Substituted bicyclo[2.1.1]hexanes (BCHs) are emerging bicyclic hydrocarbon bioisosteres for ortho- and meta-substituted benzenes, but are difficult to access. Now a SmI2-catalysed intermolecular coupling of bicyclo[1.1.0]butyl ketones and alkenes provides a general approach to access substituted BCHs, thus promoting their widespread use in medicinal chemistry and crop science.
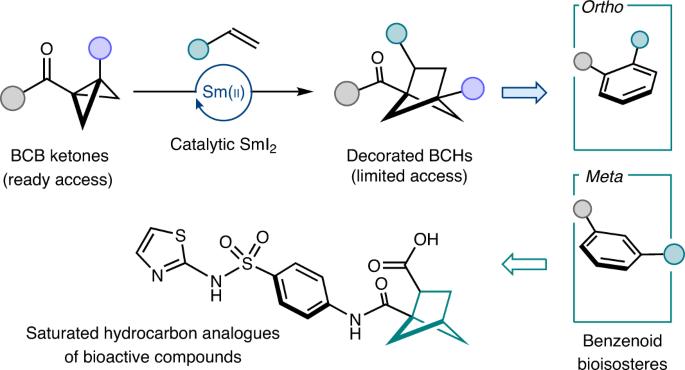
催化烯插入双环[2.1.1]己烷生物异构体的方法
以双环[1.1.1]戊烷为例,富含 C(sp3)的双环烃支架作为苯类化合物的饱和生物寄主,在药物化学和作物科学领域发挥着越来越重要的作用。取代的双环[2.1.1]己烷(BCHs)是新出现的正取代苯和偏取代苯的双环烃生物异构体,但很难获得。因此,如果要实现 BCHs 作为生物异养生物的潜力,就需要一条通向 BCHs 的合成路线。在此,我们介绍了一种广泛适用的催化方法,该方法通过烯烃和双环[1.1.0]丁基(BCB)酮之间的分子间偶联来提供取代的 BCHs。SmI2 催化的工艺适用于多种缺电子烯烃和取代的 BCB 酮,SmI2 负载低至 5 摩尔% 即可运行,其基础是得到密度泛函理论计算支持的自由基中继机制。通过选择性下游操作和快速合成广谱抗菌剂酞磺胺噻唑的饱和碳氢化合物类似物,证明了 BCH 酮产物是多功能合成中间体。取代的双环[2.1.1]己烷(BCHs)是正取代苯和偏取代苯的新兴双环烃生物异构体,但很难获得。现在,SmI2 催化的双环[1.1.0]丁基酮与烯烃的分子间偶联为获得取代的 BCHs 提供了一种通用方法,从而促进了它们在药物化学和作物科学中的广泛应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


