Diversifying Resistance Mechanisms in Cereal Crops Using Microphenomics.
IF 7.6
1区 农林科学
Q1 AGRONOMY
引用次数: 0
Abstract
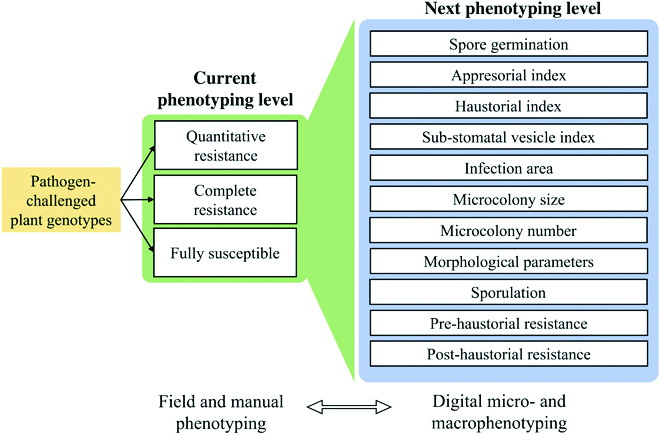
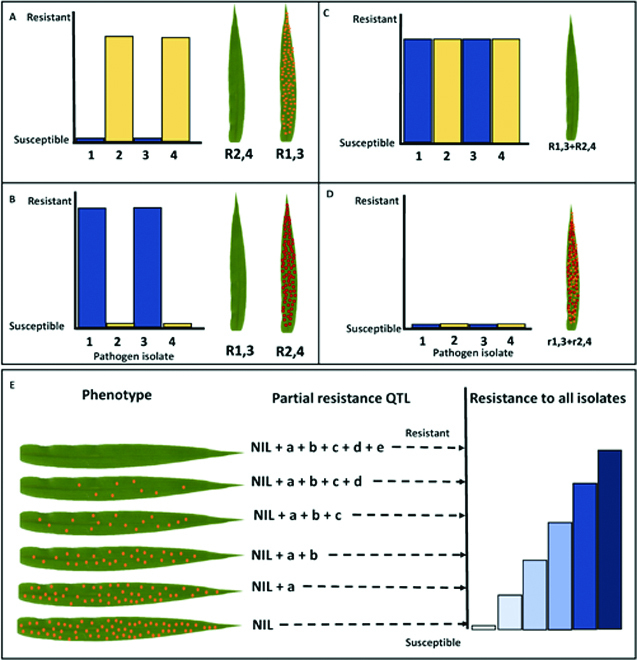
Whole-grain cereals, including wheat, barley, oat, rye, corn, rice, millet, and triticale, are a rich source of calories, essential vitamins, minerals, and phytochemicals that both nourish and protect humans and animals from diseases such as heart attack and cancer [1]. However, susceptibility to foliar diseases caused by necrotrophic or biotrophic fungal pathogens continues to reduce yield potential or lead to total crop failure and famine in developing nations [2]. Historically, foliar diseases of cereals have been controlled using either fungicide treatment [3] or plant breeding [4]. However, fungicides create selection pressure in favor of the emergence of insensitive pathogen variants and are both expensive and harmful to human health and the environment. In contrast, deploying disease resistance genes in improved cereal varieties has proven the most economical and environmentally sustainable approach to protect yield potential and ensure adequate quantities of pesticide-free food [5]. Plant genotypes or populations exposed to pathogenic microbes often vary in their phenotypic response or degree of infestation, and this is due to differences in inherited defenses. Plant breeders and geneticists are constantly assessing visual symptoms of disease resistance traits in their experimental material. Although these data are valuable, its reproducibility is limited due to individual scoring biases, non-quantitative inoculation methods, and environmental variables. Currently, there is an imbalance between our ability to manipulate plant genomes and our capacity to phenotype accurately for disease, creating a bottleneck in the plant breeding pipeline. Despite next-generation sequencing reducing genotyping costs by more than 100-fold in the last 20 years, the cost and inaccuracy of disease phenotyping have impeded genetic gain. Furthermore, the requirement of phenotypic accuracy for trait dissection and breeding has led to a historic selection bias towards race-specific resistance genes of major phenotypic effect that are easier to phenotype but rarely durable in agricultural settings when deployed singly, due to rapidly evolving pathogen populations [6] (Fig. 1A to D). In contrast, partial resistance (PR) refers to a reduction or delayed growth of the pathogen and is typically conditioned by several quantitatively inherited alleles (Fig. 1E). This form of resistance is durable, non-race-specific, and incomplete when considering the effect of a single locus in isolation [7]. However, the cumulative effect of several partial minor resistance loci often leads to complete immunity and has been reported to account for reduced disease epidemics. Both gene isolation and subsequent functional characterization studies in cereal crop plants demonstrate that PR genes show higher mechanistic diversification relative to the R genes explaining their durability [8–10]. For convenience, the term adult plant resistance (APR) is commonly used to describe PR observed in the field [11,12]. Field phenotyping for PR or APR is currently the major bottleneck in breeding for durable resistance as, in most cases, assessment can only be performed annually and is affected by environmental inconsistencies reducing phenotypic resolution. Furthermore, PR at the seedling stage can only be analyzed using a combination of uniform inoculation (use of settling tower) and quantitative manual assessments of traits, including infection frequency and delayed latency period, and therefore goes undetected using non-quantitative inoculation and phenotyping methods [13]. Visible macroscopic infection phenotypes provide an intuitive estimation of the disease resistance that has been traditionally utilized by breeders and researchers with experience in analyzing field trial data. However, the infection ratings typically represent the end point of the plant–pathogen interactions and deliver no information about the early and intermediate steps and involved resistance mechanisms. This approach also often combines resistance responses with entirely different backgrounds, thus dramatically lowering the sensitivity and resolution of genome-wide association scans (GWAS). Pathogen invasion is usually associated with rapid and dramatic gene expression, metabolism, and biochemistry changes [14–16]. Therefore, splitting the observation of the plant–pathogen interactions into a time series, starting from very early time points, may provide valuable information on the infection process and plant responses, especially when combined with different data sources, like transcriptomics and metabolomics. The enormous progress in digital image analysis achieved over the last few decades has enabled a new level of phenotyping based on processing large amounts of complex image data. Furthermore, artificial intelligence methods, such as machine learning (ML) and deep learning (DL), have been Citation: Dracatos PM, Lück S, Douchkov DK. Diversifying Resistance Mechanisms in Cereal Crops Using Microphenomics. Plant Phenomics 2023;5:Article 0023. https://doi. org/10.34133/plantphenomics.0023
利用微量表型组学研究谷类作物的抗性机制
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Plant Phenomics
Multiple-
CiteScore
8.60
自引率
9.20%
发文量
26
审稿时长
14 weeks
期刊介绍:
Plant Phenomics is an Open Access journal published in affiliation with the State Key Laboratory of Crop Genetics & Germplasm Enhancement, Nanjing Agricultural University (NAU) and published by the American Association for the Advancement of Science (AAAS). Like all partners participating in the Science Partner Journal program, Plant Phenomics is editorially independent from the Science family of journals.
The mission of Plant Phenomics is to publish novel research that will advance all aspects of plant phenotyping from the cell to the plant population levels using innovative combinations of sensor systems and data analytics. Plant Phenomics aims also to connect phenomics to other science domains, such as genomics, genetics, physiology, molecular biology, bioinformatics, statistics, mathematics, and computer sciences. Plant Phenomics should thus contribute to advance plant sciences and agriculture/forestry/horticulture by addressing key scientific challenges in the area of plant phenomics.
The scope of the journal covers the latest technologies in plant phenotyping for data acquisition, data management, data interpretation, modeling, and their practical applications for crop cultivation, plant breeding, forestry, horticulture, ecology, and other plant-related domains.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


