Retinal pathological features and proteome signatures of Alzheimer’s disease
Abstract
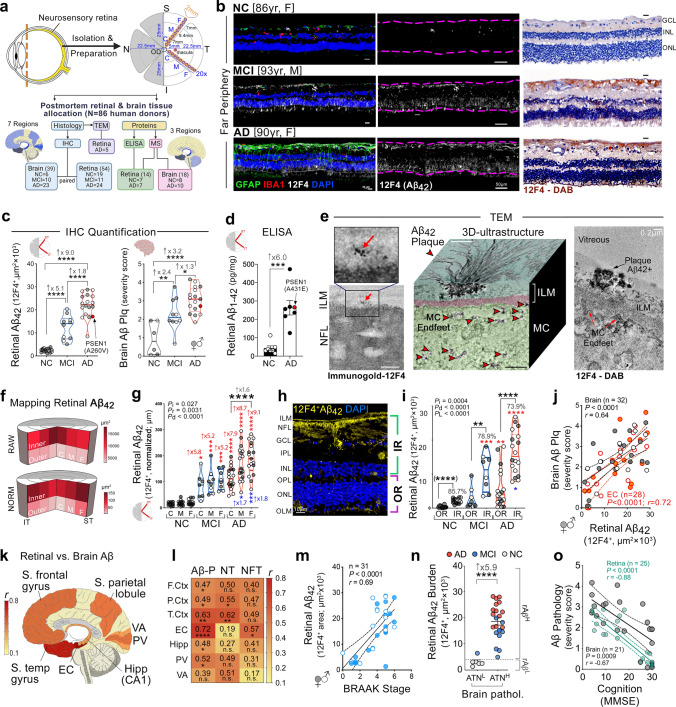
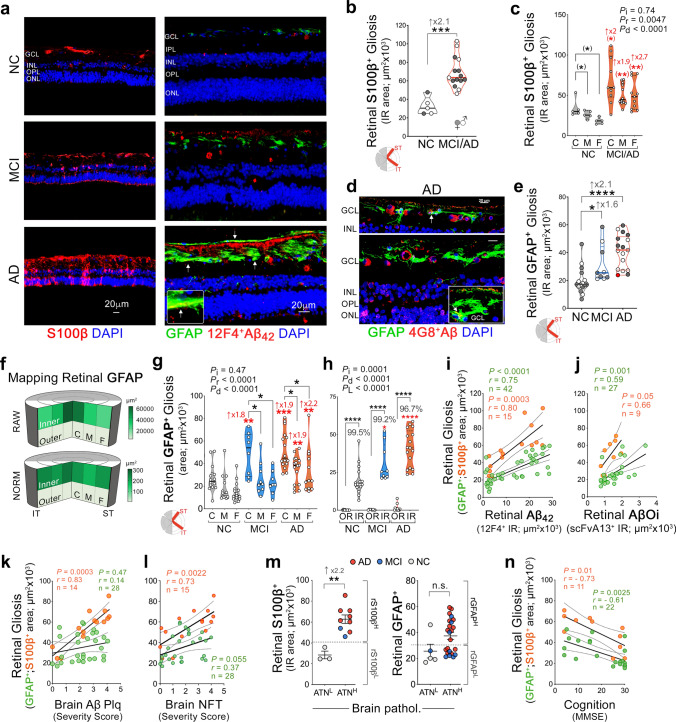
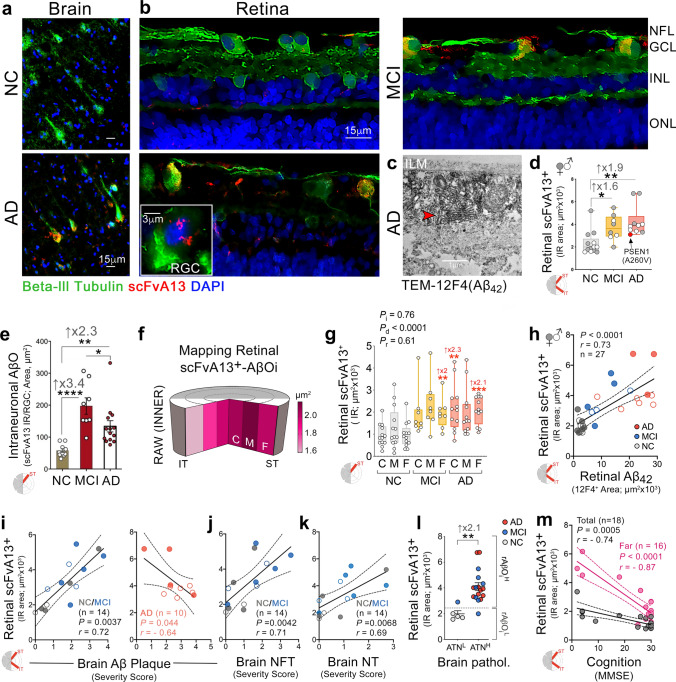
Alzheimer’s disease (AD) pathologies were discovered in the accessible neurosensory retina. However, their exact nature and topographical distribution, particularly in the early stages of functional impairment, and how they relate to disease progression in the brain remain largely unknown. To better understand the pathological features of AD in the retina, we conducted an extensive histopathological and biochemical investigation of postmortem retina and brain tissues from 86 human donors. Quantitative examination of superior and inferior temporal retinas from mild cognitive impairment (MCI) and AD patients compared to those with normal cognition (NC) revealed significant increases in amyloid β-protein (Aβ42) forms and novel intraneuronal Aβ oligomers (AβOi), which were closely associated with exacerbated retinal macrogliosis, microgliosis, and tissue atrophy. These pathologies were unevenly distributed across retinal layers and geometrical areas, with the inner layers and peripheral subregions exhibiting most pronounced accumulations in the MCI and AD versus NC retinas. While microgliosis was increased in the retina of these patients, the proportion of microglial cells engaging in Aβ uptake was reduced. Female AD patients exhibited higher levels of retinal microgliosis than males. Notably, retinal Aβ42, S100 calcium-binding protein B+ macrogliosis, and atrophy correlated with severity of brain Aβ pathology, tauopathy, and atrophy, and most retinal pathologies reflected Braak staging. All retinal biomarkers correlated with the cognitive scores, with retinal Aβ42, far-peripheral AβOi and microgliosis displaying the strongest correlations. Proteomic analysis of AD retinas revealed activation of specific inflammatory and neurodegenerative processes and inhibition of oxidative phosphorylation/mitochondrial, and photoreceptor-related pathways. This study identifies and maps retinopathy in MCI and AD patients, demonstrating the quantitative relationship with brain pathology and cognition, and may lead to reliable retinal biomarkers for noninvasive retinal screening and monitoring of AD.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


