Metal-Free, Rapid, and Highly Chemoselective Reduction of Aromatic Nitro Compounds at Room Temperature
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 15
Abstract
In this study, we developed a metal-free and highly chemoselective method for the reduction of aromatic nitro compounds. This reduction was performed using tetrahydroxydiboron [B2(OH)4] as the reductant and 4,4′-bipyridine as the organocatalyst and could be completed within 5 min at room temperature. Under optimal conditions, nitroarenes with sensitive functional groups, such as vinyl, ethynyl, carbonyl, and halogen, were converted into the corresponding anilines with excellent selectivity while avoiding the undesirable reduction of the sensitive functional groups.
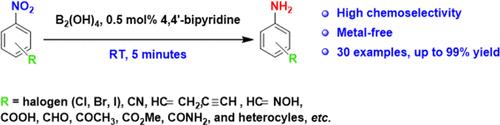
室温下无金属、快速、高化学选择性的芳香族硝基化合物还原
在这项研究中,我们开发了一种无金属和高化学选择性的方法来还原芳香硝基化合物。该还原反应以四羟基二硼[B2(OH)4]为还原剂,4,4′-联吡啶为有机催化剂,在室温下5分钟内完成。在最优条件下,具有敏感官能团(乙烯基、乙基、羰基和卤素)的硝基芳烃以极好的选择性转化为相应的苯胺,同时避免了敏感官能团的不良还原。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


