Molecular modeling of 1-butyl-3-methylimidazolium based ionic liquids for potential applications in the desulfurization of diesel fuel
Abstract
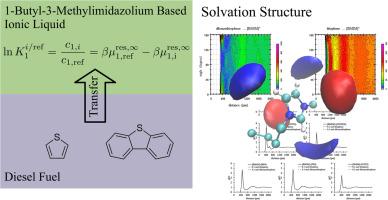
The sulfur compounds in diesel fuel produce harmful environmental pollutants during combustion. Hydrodesulfurization (HDS) is the most common technique to reduce the sulfur content of diesel fuel but cannot effectively remove aromatic sulfur compounds to produce ultra-low-sulfur diesel. Ionic liquids (ILs) show potential as alternative solvents for extractive desulfurization to be implemented after a conventional HDS process, but the mechanism is not well understood. This work focuses on using a combination of molecular simulation free energy calculations and detailed structural analysis to better understand the molecular-level interactions between dibenzothiophene and seven common imidazolium-based ILs. The free energy calculations suggest that the ILs interact differently with thiophene and dibenzothiophene. No specific interactions were observed between the anion and dibenzothiophene; varying the anion showed no remarkable differences in the observed interactions. It was determined that interactions between dibenzothiophene and the cation were more significant; π-π stacking between the imidazole ring and thiophene ring plus favorable electrostatic interactions between the alkyl chain and benzene rings were observed. The primary goal of this work is to use molecular simulations to complement current experimental research to identify suitable ILs for potential extractive desulfurization applications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


