SPI1 Mediates N-Myristoyltransferase 1 to Advance Gastric Cancer Progression via PI3K/AKT/mTOR Pathway.
IF 2.3
4区 医学
Q2 Medicine
引用次数: 0
Abstract
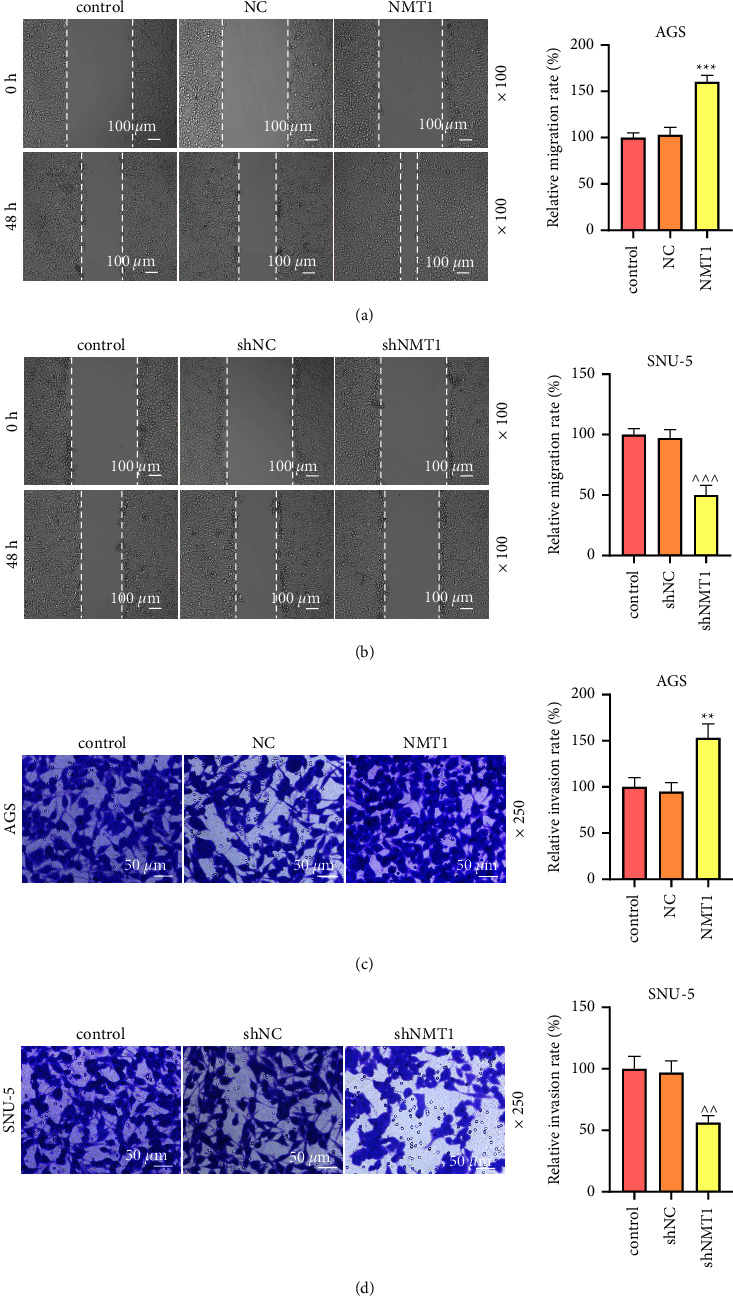
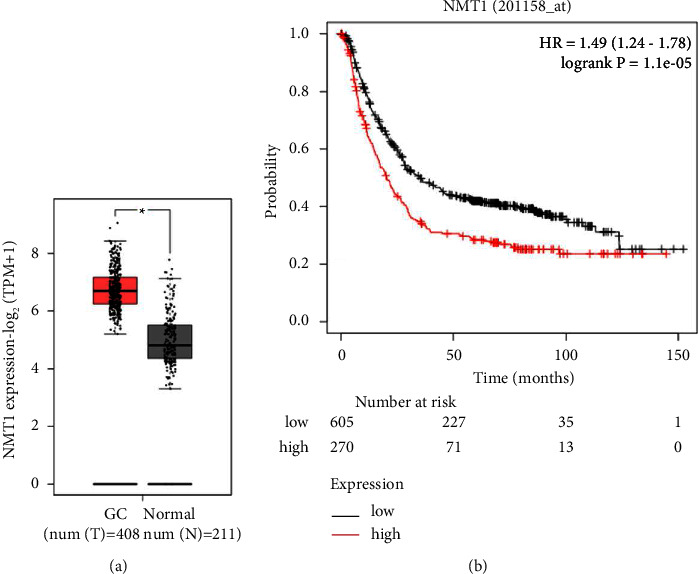
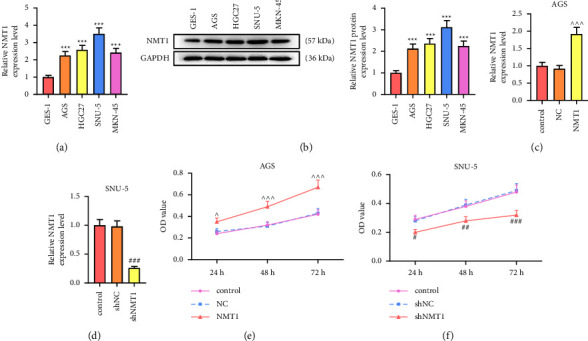
Gastric cancer (GC) is a common digestive tract malignancy worldwide. N-myristoyltransferase 1 (NMT1) has been implicated in many cancers, but its association with gastric cancer remains to be clarified. Thus, this paper elucidated the role of NMT1 in GC. The NMT1 expression level in GC and normal tissue samples as well as the relationship between NMT1 high or low expression and overall survival in GC was analyzed via GEPIA. GC cells were transfected with NMT1 or SPI1 overexpression plasmid and short hairpin RNA against NMT1 (shNMT1) or shSPI1. NMT1, SPI1, p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR levels were detected through qRT-PCR and western blot. MTT, wound healing, and transwell assays were applied to test cell viability, migration, and invasion. The binding relationship of SPI1 and NMT1 was determined through a dual-luciferase reporter assay and chromatin immunoprecipitation. NMT1 was upregulated in GC, the high level of which connected with a poor prognosis. Overexpressed NMT1 elevated viability, migration rate, and invasion rate of GC cells, whereas NMT1 knockdown leads to the opposite results. Besides, SPI1 could bind to NMT1. Overexpressed NMT1 reversed the effects of shSPI1 on decreasing viability, migration, invasion, p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR in GC cells, and NMT1 knockdown reversed the effects of SPI1 overexpression on increasing viability, migration, invasion, p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR. SPI1 upregulated NMT1 to facilitate the malignant behaviors of GC cells through the PI3K/AKT/mTOR pathway.
SPI1介导n -肉豆蔻酰基转移酶1通过PI3K/AKT/mTOR通路促进胃癌进展
胃癌是一种常见的消化道恶性肿瘤。n -肉豆芽酰基转移酶1 (NMT1)与许多癌症有关,但其与胃癌的关系尚不清楚。因此,本文阐明了NMT1在GC中的作用。通过GEPIA分析NMT1在胃癌和正常组织样本中的表达水平,以及NMT1高表达或低表达与胃癌总生存率的关系。用NMT1或SPI1过表达质粒和短发夹RNA转染GC细胞,对抗NMT1 (shNMT1)或shSPI1。采用qRT-PCR和western blot检测NMT1、SPI1、p-PI3K、PI3K、p-AKT、AKT、p-mTOR、mTOR水平。MTT、伤口愈合和transwell试验用于检测细胞活力、迁移和侵袭。SPI1和NMT1的结合关系是通过双荧光素酶报告试验和染色质免疫沉淀来确定的。NMT1在GC中表达上调,其高水平与预后不良有关。过表达NMT1可提高GC细胞的活力、迁移率和侵袭率,而敲低NMT1则会导致相反的结果。此外,SPI1可以与NMT1结合。NMT1过表达逆转了shSPI1降低GC细胞活力、迁移、侵袭、p-PI3K/PI3K、p-AKT/AKT和p-mTOR/mTOR的作用,NMT1敲低逆转了SPI1过表达提高GC细胞活力、迁移、侵袭、p-PI3K/PI3K、p-AKT/AKT和p-mTOR/mTOR的作用。SPI1通过PI3K/AKT/mTOR通路上调NMT1,促进GC细胞的恶性行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Canadian Journal of Gastroenterology and Hepatology
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
4.80
自引率
0.00%
发文量
0
审稿时长
37 weeks
期刊介绍:
Canadian Journal of Gastroenterology and Hepatology is a peer-reviewed, open access journal that publishes original research articles, review articles, and clinical studies in all areas of gastroenterology and liver disease - medicine and surgery.
The Canadian Journal of Gastroenterology and Hepatology is sponsored by the Canadian Association of Gastroenterology and the Canadian Association for the Study of the Liver.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


