An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 32
Abstract
Bicyclic hydrocarbons, and bicyclo[1.1.1]pentanes (BCPs) in particular, are playing an emerging role as saturated bioisosteres in pharmaceutical, agrochemical and materials chemistry. Taking advantage of strain-release strategies, prior synthetic studies have featured the synthesis of bridgehead-substituted (C1, C3) BCPs from [1.1.1]propellane. Here, we describe an approach to access multisubstituted BCPs via intramolecular cyclization. In addition to C1,C3-disubstituted BCPs, this method also enables the construction of underexplored multisubstituted (C1, C2 and C3) BCPs from readily accessible cyclobutanones. The broad generality of this method has also been examined through the synthesis of a variety of other caged bicyclic molecules, ranging from [2.1.1] to [3.2.1] scaffolds. The modularity afforded by the pendant bridgehead boron pinacol esters generated during the cyclization reaction has been demonstrated through several downstream functionalizations, highlighting the ability of this approach to enable the programmed and divergent synthesis of multisubstituted bicyclic hydrocarbons. Bicyclic hydrocarbons, and bicyclo[1.1.1]pentanes in particular, are playing an emerging role as saturated bioisosteres in pharmaceutical, agrochemical and materials intramolecular coupling approach has been developed for the modular construction of underexplored multisubstituted strained bicyclic hydrocarbons, ranging from [1.1.1] to [3.2.1] scaffolds.
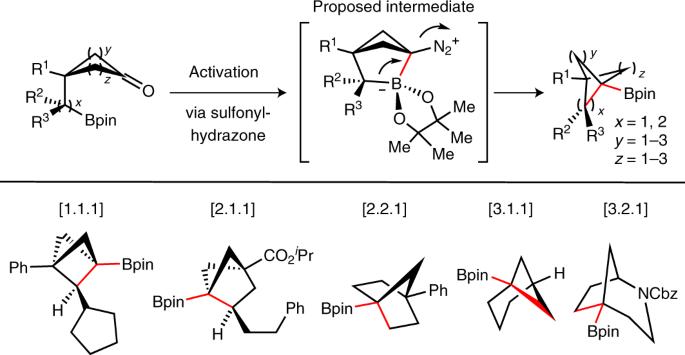
分子内偶联烷基生物异构体合成多取代双环烷基硼酸盐的方法
双环烃类,尤其是双环[1.1.1]戊烷(BCPs),作为饱和生物异构体,正在医药、农用化学品和材料化学领域发挥着越来越重要的作用。利用应变释放策略,之前的合成研究以从 [1.1.1]propellane 合成桥头取代 (C1, C3) BCPs 为特色。在此,我们介绍一种通过分子内环化获得多取代 BCP 的方法。除了 C1、C3-二取代 BCP 外,这种方法还能从容易获得的环丁酮中构建尚未充分开发的多取代(C1、C2 和 C3)BCP。通过合成从[2.1.1]到[3.2.1]支架的各种其他笼状双环分子,我们还考察了这种方法的广泛通用性。环化反应过程中生成的桥头硼频哪醇酯的模块化特性已通过几种下游官能化反应得到证实,突出表明这种方法能够实现多取代双环烃的程序化和发散合成。双环烃,特别是双环[1.1.1]戊烷,作为饱和生物异质材料,在制药、农用化学品和材料领域发挥着新兴作用。我们开发了分子内偶联方法,用于模块化构建尚未充分开发的多取代受限双环烃,范围从[1.1.1]到[3.2.1]支架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


