Frustrated Lewis pair catalyzed C–F activation of α-trifluoromethylstyrenes
引用次数: 0
Abstract
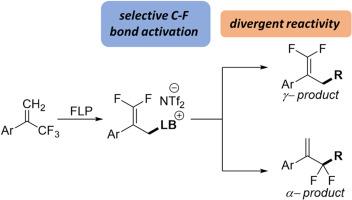
A frustrated Lewis pair approach is used for the monoselective C–F activation of α-trifluoromethyl styrenes. Selective C–F activation of α-trifluoromethyl styrenes with trispentafluorophenylborane (BCF) in partnership with tri(ortho-tolyl)phosphine or 2,4,6-triphenylpyridine (TPPy) generates γ-substituted α,α-difluoropropenyl phosphonium or pyridinium salts (respectively). The ability to further functionalize such products at the α and γ-positions is demonstrated through nucleophilic substitutions and metal catalyzed Suzuki couplings to generate a range of difluoromethyl and 1,1-difluoroolefin products.
受挫Lewis对催化α-三氟甲基苯乙烯的C-F活化
受挫路易斯对方法用于α-三氟甲基苯乙烯的单选择性C-F活化。用三五氟苯硼烷(BCF)与三(邻苯二甲酸)膦或2,4,6-三苯基吡啶(TPPy)选择性C-F活化α-三氟甲基苯乙烯(分别生成γ-取代α、α-二氟丙烯磷或吡啶盐)。通过亲核取代和金属催化的铃木偶联,证明了在α和γ-位置进一步功能化这些产品的能力,从而产生一系列二氟甲基和1,1-二氟烯烃产品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


