The gut–joint axis in rheumatoid arthritis
IF 29.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 118
Abstract
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disorder that primarily affects the joints. One hypothesis for the pathogenesis of RA is that disease begins at mucosal sites as a consequence of interactions between the mucosal immune system and an aberrant local microbiota, and then transitions to involve the synovial joints. Alterations in the composition of the microbial flora in the lungs, mouth and gut in individuals with preclinical and established RA suggest a role for mucosal dysbiosis in the development and perpetuation of RA, although establishing whether these alterations are the specific consequence of intestinal involvement in the setting of a systemic inflammatory process, or whether they represent a specific localization of disease, is an ongoing challenge. Data from mouse models of RA and investigations into the preclinical stages of disease also support the hypothesis that these alterations to the microbiota predate the onset of disease. In addition, several therapeutic options widely used for the treatment of RA are associated with alterations in intestinal microbiota, suggesting that modulation of intestinal microbiota and/or intestinal barrier function might be useful in preventing or treating RA. Intestinal dysbiosis is thought to be involved in the early stages of rheumatoid arthritis (RA). In this Review, the authors discuss the gut–joint axis in RA and the potentially pathogenic role of gut-derived immune cells in the joints.
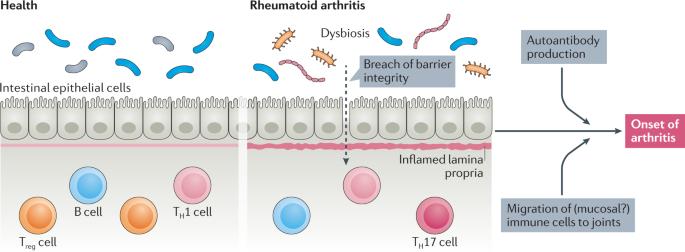
类风湿关节炎的肠道关节轴
类风湿性关节炎(RA)是一种主要影响关节的慢性自身免疫性炎症性疾病。关于类风湿性关节炎发病机制的一种假设是,疾病始于粘膜部位,是粘膜免疫系统与异常的局部微生物群相互作用的结果,然后过渡到累及滑膜关节。临床前和已确诊的 RA 患者肺部、口腔和肠道中微生物菌群组成的改变表明,粘膜菌群失调在 RA 的发展和持续中起着一定的作用,但确定这些改变是全身炎症过程中肠道受累的特定结果,还是代表疾病的特定定位是一个持续的挑战。来自 RA 小鼠模型的数据和对疾病临床前阶段的调查也支持这样的假设,即微生物群的这些改变发生在疾病发作之前。此外,广泛用于治疗 RA 的几种治疗方案都与肠道微生物群的改变有关,这表明调节肠道微生物群和/或肠道屏障功能可能有助于预防或治疗 RA。肠道菌群失调被认为与类风湿性关节炎(RA)的早期阶段有关。在这篇综述中,作者讨论了类风湿关节炎的肠道-关节轴以及肠道免疫细胞在关节中的潜在致病作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


