A sustainable synthesis of 3,3-disubstituted oxindoles via CuBr-catalysed capture of carboxylic oxonium ylides with isatylidene malononitrile
引用次数: 0
Abstract
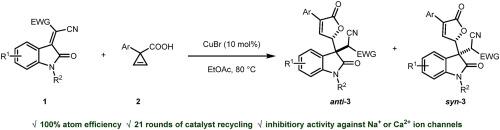
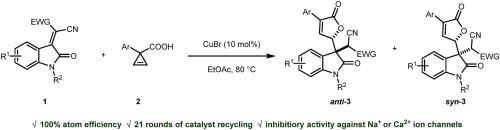
We herein reported a sustainable synthesis of 3,3-disubstituted oxindoles via a Michael-type reaction based on the CuBr-catalysed capture of carboxylic oxonium ylides with isatylidene malononitrile. The reaction is characterized by a high atom economy and low economic constraints. The catalyst CuBr could be conveniently recyclized. The products of the reaction were found to be inhibitory against Na ion channels. We expect the reaction to shed the light on synthesis of biologically interesting molecules directed by principles of green chemistry.
通过 CuBr 催化异亚甲基丙二腈捕获羧基氧鎓酰化物可持续合成 3,3-二取代吲哚
我们在此报告了一种可持续的 3,3-二取代吲哚的合成方法,该方法基于 CuBr 催化的羧基氧鎓酰化物与异亚甲基丙二腈的捕获,通过迈克尔型反应进行合成。该反应的特点是原子经济性高,经济限制低。催化剂 CuBr 可以方便地回收利用。该反应的产物对 Na 离子通道具有抑制作用。我们期待该反应能为利用绿色化学原理合成具有生物学意义的分子提供启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


