Highly enantio-stereoselective Ni-catalyzed reductive cyclization to cyclopentanes with chiral quaternary centres of trisubstituted allylic siloxanes
引用次数: 0
Abstract
The construction of chiral quaternary carbon stereocenters is a significant challenge in asymmetric synthesis. Catalytic synthesis of these structures with trisubstituted allylic alcohols is highly important. However, most of the reported methodologies required noble transition-metals. Herein we reported the first highly asymmetric stereoselective synthesis of cyclopentanes bearing chiral quaternary carbon stereocenters of trisubstituted allylic siloxanes by reductive cyclization of all carbon 1,6-alkynones with the non-noble nickel catalysis of Ni(cod)2 with P-chiral monophosphine ligand (S)-BIDIME. Various multi-substituted functionalized cyclopentanes were obtained in high yields (up to 96%), excellent enantioselectivities (up to >99% ee) and perfect stereoselectivities (>99:1 E/Z). Thirty-two examples were successfully established for this method. Clarified mechanism studies were investigated first time by React-IR and DFT calculations to understand and explain the ligand-control of excellent enantio-stereoselectivity. Gram-scale reaction and control experiments were carried out. A new reaction design was proposed for further application of this type of catalysis.
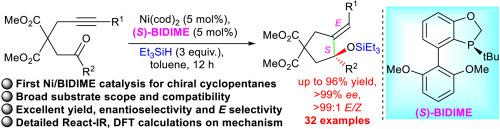
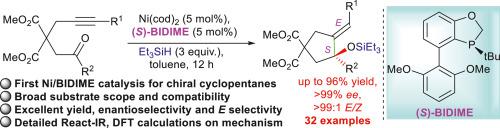
高对映立体选择性镍催化的三取代烯丙基硅氧烷手性季中心环戊烷还原环化反应
手性季碳立体中心的构造是不对称合成中的一个重大挑战。用三取代烯丙醇催化合成这些结构是非常重要的。然而,大多数报告的方法都需要贵重的过渡金属。本文报道了在Ni(cod)2和p -手性单膦配体(S)-BIDIME的非贵金属镍催化下,通过1,6-炔酮全碳还原环化,首次合成了具有手性季碳立体中心的三取代烯丙基硅氧烷的高度不对称立体选择性环戊烷。以高收率(96%)、优异的对映选择性(99% ee)和良好的立体选择性(99:1 E/Z)得到了多种多取代功能化环戊烷。该方法成功建立了32个算例。通过反应- ir和DFT计算,首次研究了明确的机理,以了解和解释配体对良好的对映立体选择性的控制。进行了克级反应和对照实验。提出了一种新的反应设计,以进一步应用这种类型的催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


