Development of a Scalable Route toward an Alkylated 1,2,4-Triazol, a Key Starting Material for CXCR3 Antagonist ACT-777991
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 2
Abstract
Several synthetic routes toward triazole building block 2-(3-methyl-1H-1,2,4-triazol-1-yl)acetic acid are described. The main problems of the initial synthetic route via alkylation of 3-Me-1H-1,2,4-triazole, such as poor regioselectivity, low yield, and purification by column chromatography, could be significantly improved or completely avoided in the second-generation approaches. Key concepts for the design of the alternative synthesis approaches to solve the problem of regioselectivity were the desymmetrization of 3,5-dibromo-1H-1,2,4-triazole and the de novo synthesis of the triazole core. The scalability of all routes was demonstrated on >100 g scale.
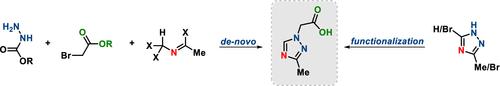
CXCR3拮抗剂ACT-777991关键起始原料烷基化1,2,4-三唑的可扩展路线的发展
介绍了几种合成三唑基2-(3-甲基- 1h -1,2,4-三唑-1-基)乙酸的方法。初始3- me - 1h -1,2,4-三唑烷基化合成路线存在区域选择性差、收率低、柱层析纯化等主要问题,在第二代方法中可以显著改善或完全避免。设计解决区域选择性问题的替代合成方法的关键概念是3,5-二溴- 1h -1,2,4-三唑的不对称和三唑核的重新合成。在100g规模上验证了所有路由的可扩展性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


