Differentiation of Chemical Components in Basaltic Melts During Eruptions: An Example from Tolbachik–Hawaiian Fissure Zones and Etna Vent
Abstract
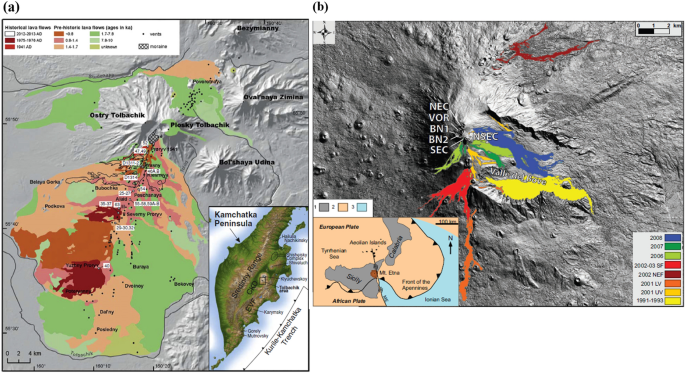
The study on differentiation of chemical components in quenched volcanics from the Etna, Tolbachik and Hawaiian fissure zones was carried out under simulated temperature/pressure (T/P) conditions reflecting real-time conditions in the early stages of magmatic eruptions. The simulation was performed under high-pressure chamber apparatus, and the rocks were then subjected to high T/P conditions resulting in basaltic melts. Various temperature and pressure conditions were adjusted from the apparatus to allow for different rates of magmatic cooling and crystallization. The scanning electron microscopy (SEM) coupled with energy-dispersive spectroscopy (EDS-microanalysis) was then used to study the quenched glasses with the aim of identifying major element partitioning based on micro-heterogeneity of melts structures. The microscopic study of the experimental melts and their comparisons with natural melts under the electron microscopes indicate the existence of two distinct liquids which fractionated during cooling on the basis of their chemical composition (compositional melts): Fe, Mg liquids concentrated in the poorly polymerized and mobile parts of the melt and K, Na and Al liquids were concentrated in more highly polymerized parts of the melts. Partitioning of Ca and Si appears to be changeable and more dependent on bulk melt composition. Fractionation of non-crystalline melts was evident under post-explosion sharp quenching and high-pressure conditions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


