Chemoselective synthesis of α-carboline derivatives via hypervalent iodine-catalyzed [3+3] annulation under metal-free conditions
引用次数: 0
Abstract
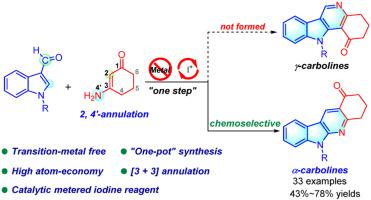
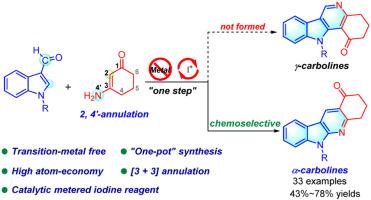
A strategy for the synthesis of α-carboline derivatives from indole-3-carboxaldehydes and 3-aminocyclohex-2-enones under metal-free conditions has been developed. The combination use of phenyliodine (III) diacetate (PIDA) and benzoic acid could significantly facilitate the corresponding [3 + 3] annulation process. This newly developed strategy featured unextraordinary chemoselectivity, good functional group tolerance and the preservation of the carbonyl group of the ketone substrates, which offers the possibility for further transformation of the products.
在无金属条件下通过高价碘催化 [3+3] 环化化学选择性合成 α-咔啉衍生物
我们开发了一种在无金属条件下从吲哚-3-甲醛和 3-氨基环己-2-烯酮合成 α-咔啉衍生物的策略。苯碘 (III) 二乙酸酯 (PIDA) 和苯甲酸的结合使用大大促进了相应的 [3 + 3] 环化过程。这种新开发的策略具有非凡的化学选择性、良好的官能团耐受性以及保留了酮基底物的羰基,从而为产物的进一步转化提供了可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


