Visible-light induced photocatalyst-free difluoromethylation of quinoxalinones with difluorosulfones
引用次数: 0
Abstract
Visible-light induced direct C–H difluoromethylation of quinoxalinones with 2-((difluoromethyl)sulfonyl)benzo[d]thiazole has been developed for the first time. A broad range of quinoxalinones were well tolerated and reacted with difluorosulfone smoothly to give the corresponding products in moderate to good yields. Notably, no external photocatalyst or oxidant was required, which provides a practical and green protocol to access difluoromethylated quinoxalinones. Finally, the control experiments demonstrated a radical mechanism, and the density functional theory (DFT) calculations indicated the radicals were generated through the formation of an electron donor−acceptor (EDA) complex.
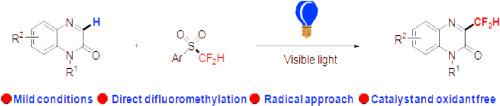
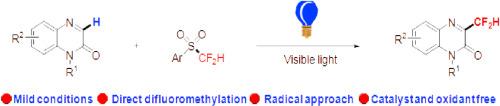
可见光诱导的无光催化剂喹啉酮与二氟砜的二氟甲基化
本文首次报道了喹啉酮类化合物与2-((二氟甲基)磺酰)苯并[d]噻唑在可见光诱导下的直接C-H二氟甲基化反应。多种喹诺沙林酮具有良好的耐受性,与二氟砜反应平稳,产率中等至较高。值得注意的是,不需要外部光催化剂或氧化剂,这为获取二氟甲基化喹诺沙林酮提供了一种实用和绿色的方案。最后,对照实验证明了自由基机制,密度泛函理论(DFT)计算表明自由基是通过电子供体-受体(EDA)复合物的形成而产生的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


