Photocatalytic generation of 1,4-disubstituted 1,2,3-triazoles under metal, oxidant and azide-free conditions
引用次数: 0
Abstract
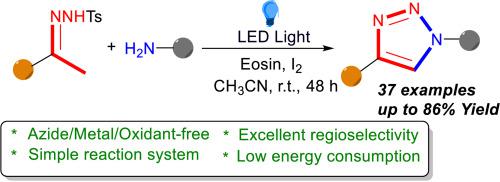
Available online A mild and efficient photocatalytic-induced radical method has been developed for [4 + 1] cycloaddition reaction of 1,4-disubstituted 1,2,3-triazoles with N-tosylhydrazides and primary amines. The reaction is catalyzed by 20 mol% of I2 under metal, azide and oxidant-free conditions. The method is based upon the photocatalytic generation of allyl-type radicals, followed by the iodine-catalyzed production of azoalkenes, which react rapidly with various anilines.
在无金属、氧化剂和叠氮化物条件下光催化生成 1,4-二取代的 1,2,3-三唑
在线提供 一种温和高效的光催化诱导自由基方法已被开发出来,用于 1,4 二取代的 1,2,3 三唑与 N-对甲苯磺酰肼和伯胺的 [4 + 1] 环加成反应。该反应在无金属、叠氮化物和氧化剂的条件下,由 20 mol% 的 I2 催化。该方法以光催化生成烯丙基自由基为基础,然后在碘催化下生成偶氮烯,并迅速与各种苯胺发生反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


