Quantum and electrochemical studies of the hydrogen evolution findings in corrosion reactions of mild steel in acidic medium
IF 2.6
Q3 ENERGY & FUELS
引用次数: 31
Abstract
The electrochemical techniques included electrochemical impedance spectroscopy (EIS) and Tafel polarization. Changing GABA concentrations greatly impacted the rate of both the corrosion reaction and the evolution of hydrogen. The findings of polarization suggested that GABA is a mixed inhibitor of form. Rising the temperature (298–338 K) resulted in an intensification in the rate of hydrogen progression and a diminution in their steel's full superficial confrontation measure (RT) or comparative coverage width (1/CT). The inhibition capacity of GABA was demonstrated by the quantity control including EHOMO, ELUMO, the energy gap (∆E) and the segment of relocated electron (∆E).
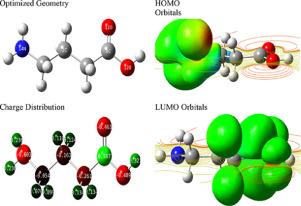
低碳钢在酸性介质中腐蚀反应中析氢结果的量子和电化学研究
电化学技术包括电化学阻抗谱(EIS)和塔菲尔极化。GABA浓度的变化极大地影响了腐蚀反应的速率和氢的析出。极化结果表明GABA是一种混合型抑制剂。升高温度(298 - 338k)导致氢的进展速度加快,钢的全表面对抗测量(RT)或相对覆盖宽度(1/CT)减小。通过对EHOMO、ELUMO、能隙(∆E)和重定位电子段(∆E)的数量控制来表征GABA的抑制能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


