Synthesis of biheteroaryls via 2-methyl quinoline C(sp3)-H functionalization under metal-free conditions
引用次数: 0
Abstract
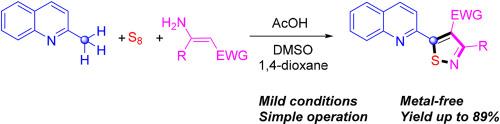
An acetic acid-promoted C(sp3)-H functionalization of 2-methyl quinoline, enaminoesters and elemental sulfur for the synthesis of 3,4,5-trisubstituted isothiazoles under metal-free conditions has been developed. This approach provides viable access to various 5-(quinolin-2-yl)isothiazoles in moderate to good yields with good functional group tolerance. Moreover, the success of the gram-scale reaction gives this reaction a great potential application.
在无金属条件下通过 2-甲基喹啉 C(sp3)-H 官能化合成生物杂芳香族化合物
本研究开发了一种乙酸促进 2-甲基喹啉、烯胺酯和元素硫的 C(sp3)-H 功能化方法,用于在无金属条件下合成 3,4,5-三取代异噻唑。这种方法提供了获得各种 5-(喹啉-2-基)异噻唑的可行途径,产率从中等到较高,并具有良好的官能团耐受性。此外,克级反应的成功也为这一反应提供了巨大的应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


