Synthesis and application of a novel monomer 5-(1-Pyrrolidinyl)-1,3-benzenedicarbonyl dichloride in membranes
Abstract
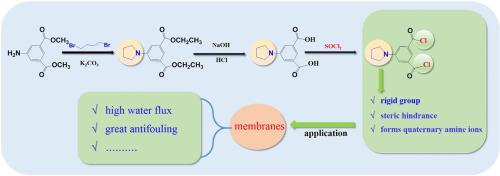
Developing novel monomers used for aromatic polyamide membranes is one of the promising modifications to tailor the membranes more efficient. Acyl chloride-based compound as the organic phase reactive monomer is vital to the fabrication of membranes. This study focuses on designing and synthesizing a novel acyl chloride monomer 5-(1-pyrrolidinyl)-1,3-benzenedicarbonyl dichloride (PIPC) based on the purpose of improving membrane permeability and anti-fouling, and preliminarily verify its feasibility for the synthesis of aromatic polyamide membranes. PIPC monomer with a rigid pyrrolidinyl group (–NC4H8) was synthesized from three steps of N-alkylation, ester hydrolysis and acylation reaction successively. IR and 1HNMR spectra were employed to demonstrate the successful synthesis of PIPC. The application of PIPC in the membrane field was also implemented via using PIPC alone as the organic phase reactive monomer, the first/second organic phase reactive monomer, and PIPC and trimesoyl chloride (TMC) together act as the organic phase reactive monomer to react with m-phenylenediamine (MPD) by interfacial polymerization (IP). The MPD-PIPC-TMC membrane prepared by PIPC as the first organic phase reactive monomer exhibited the highest water flux (27.89 L m−2 h−1), with the increase of 36.8% than the MPD-TMC membrane (20.38 L m−2 h−1), while maintaining similar salt rejection. The PIPC with a rigid pyrrolidinyl group was demonstrated to be a promising organic phase monomer for further synthesizing high permeability aromatic polyamide membrane, which showed great application prospects in the field of membrane industry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


