Heterophase Synthesis of Silver Trifluoroacetate with Copper, Indium, and Zinc. Standard Enthalpy of Formation of Copper Trifluoroacetate
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
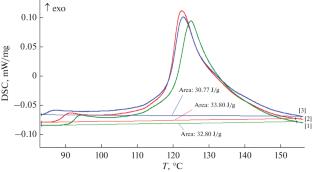
Solid-phase reactions of silver trifluoroacetate CF3COOAg with copper, indium, and zinc are studied by thermogravimetry, differential scanning calorimetry, and mass spectrometry. In a temperature range of 358–428 K, the reactions are found to afford trifluoroacetates of these metals without mass loss of the weighed samples. The obtained experimental data make it possible to calculate the enthalpy of formation of copper trifluoroacetate \({{\Delta }_{f}}H_{{298}}^{^\circ }\)(CF3СООСu, cr) = –1020.5 ± 18.0 kJ/mol.
铜、铟、锌异相合成三氟乙酸银的研究。三氟乙酸铜的标准生成焓
用热重法、差示扫描量热法和质谱法研究了三氟乙酸银CF3COOAg与铜、铟和锌的固相反应。在358–428 K的温度范围内,发现反应可以提供这些金属的三氟乙酸盐,而称重样品没有质量损失。所获得的实验数据使计算三氟乙酸铜的生成焓成为可能(CF3СООСu,cr)=–1020.5±18.0 kJ/mol。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


