Photoinduced radical generation and trapping by acrylonitrile of N-Boc secondary amine and N-Boc N-methyl α-amino acid ester at α-position using two-molecule photoredox catalysts
IF 3.261
引用次数: 0
Abstract
The photoreaction of an N-Boc secondary amine and N-Boc N-methyl α-amino acid ester with acrylonitrile using inexpensive two-molecule photoredox catalysts results in the production of α-alkylated amine through the generation of an α-carbamy radical under mild conditions. In particular, this mothed leads to a regioselective modification of N-Boc N-methyl α-amino acid ester with the retention of α-chirality through the generation of the less stable primary α-carbamyl radical.
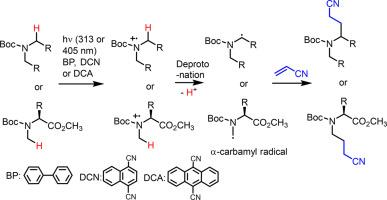
双分子光氧化还原催化剂光诱导N-Boc仲胺和N-Boc n -甲基α-氨基酸酯在α-位置的生成和被丙烯腈捕获
采用廉价的双分子光氧化还原催化剂,将N-Boc仲胺和N-Boc n -甲基α-氨基酸酯与丙烯腈进行光化学反应,在温和条件下生成α-氨基基,生成α-烷基化胺。特别是,该方法通过生成不稳定的初级α-氨基甲酰自由基,导致N-Boc n -甲基α-氨基酸酯的区域选择性修饰,保留了α-手性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


