Alternative method to Baeyer–Villiger oxidation of cyclobutenones using I2/DMSO catalytic systems†
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 1
Abstract
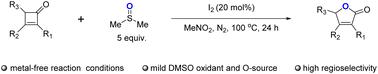
The Baeyer–Villiger oxidation represents a valuable reaction that enables the conversion of ketones into esters or lactones. Here, we present a novel and efficient approach utilizing I2 as a catalyst for the oxidative rearrangement of cyclobutenones, resulting in the synthesis of furan-2(5H)-one. Notably, this method employs dimethyl sulfoxide (DMSO) as a greener oxidant and source of oxygen. The reaction proceeds smoothly with catalytic amounts of iodine, yielding lactones in good yields. Compared to conventional Baeyer–Villiger oxidation reactions that rely on peroxyl acids or hydrogen peroxide, the use of DMSO offers a safer and more versatile alternative.
I2/DMSO催化体系对环丁烯酮Baeyer-Villiger氧化的替代方法
Baeyer-Villiger氧化反应是一种有价值的反应,可以将酮转化为酯或内酯。在这里,我们提出了一种新颖而有效的方法,利用I2作为环丁烯酮氧化重排的催化剂,合成呋喃-2(5H)- 1。值得注意的是,该方法使用二甲基亚砜(DMSO)作为更环保的氧化剂和氧气来源。在碘的催化量下,反应顺利进行,内酯产量高。与依赖过氧酸或过氧化氢的传统bayer - villiger氧化反应相比,使用DMSO提供了一种更安全、更通用的替代方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


