Immune activation state modulates infant engram expression across development
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Infantile amnesia is possibly the most ubiquitous form of memory loss in mammals. We investigated how memories are stored in the brain throughout development by integrating engram labeling technology with mouse models of infantile amnesia. Here, we found a phenomenon in which male offspring in maternal immune activation models of autism spectrum disorder do not experience infantile amnesia. Maternal immune activation altered engram ensemble size and dendritic spine plasticity. We rescued the same apparently forgotten infantile memories in neurotypical mice by optogenetically reactivating dentate gyrus engram cells labeled during complex experiences in infancy. Furthermore, we permanently reinstated lost infantile memories by artificially updating the memory engram, demonstrating that infantile amnesia is a reversible process. Our findings suggest not only that infantile amnesia is due to a reversible retrieval deficit in engram expression but also that immune activation during development modulates innate, and reversible, forgetting switches that determine whether infantile amnesia will occur.
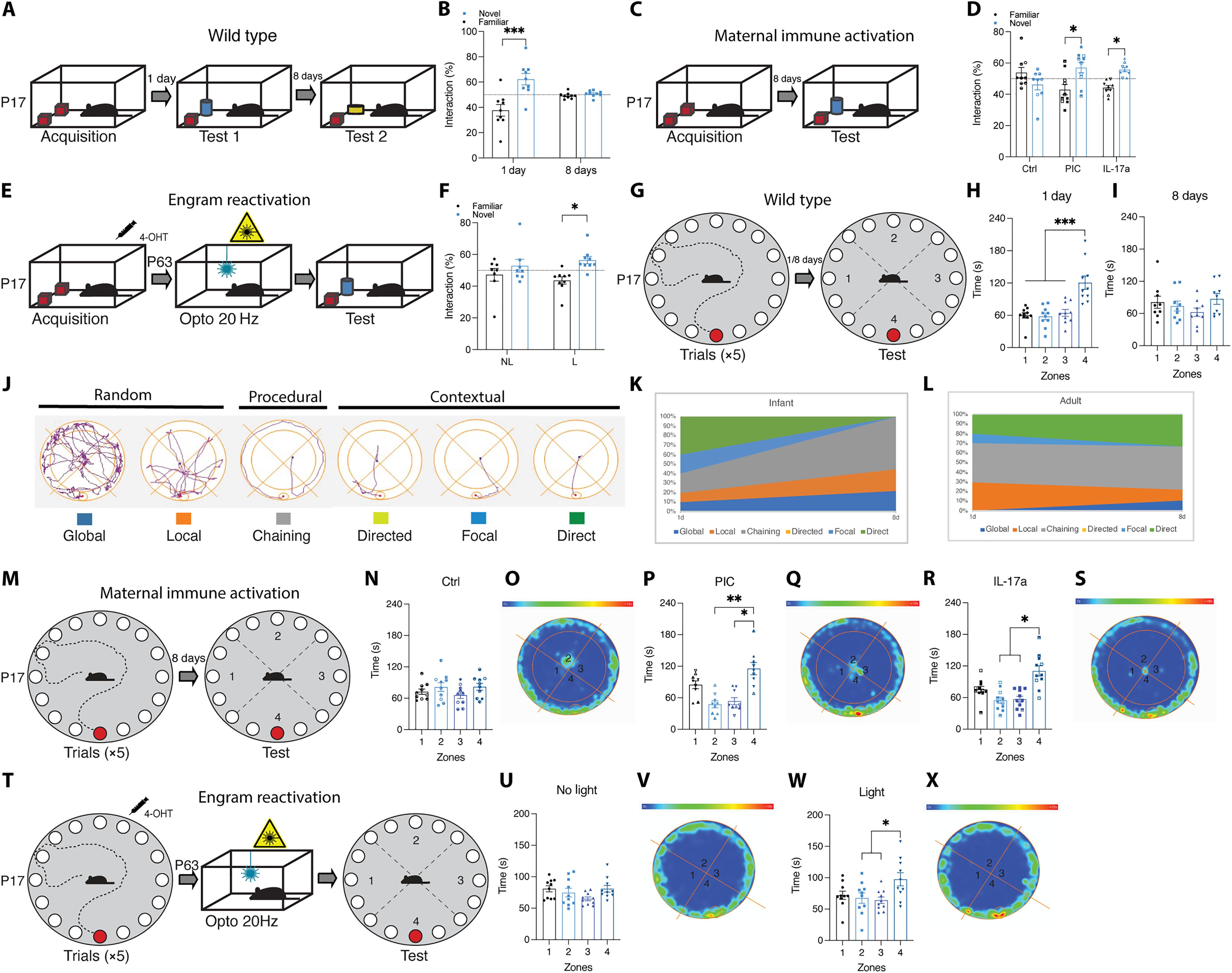
免疫激活状态在整个发育过程中调节婴儿植入物的表达。
婴儿健忘症可能是哺乳动物中最普遍的记忆丧失形式。我们通过将记忆标记技术与婴儿健忘症小鼠模型相结合,研究了记忆在整个发育过程中是如何储存在大脑中的。在这里,我们发现了一种现象,在自闭症谱系障碍的母体免疫激活模型中,男性后代不会经历婴儿健忘症。母体免疫激活改变了树突棘的整体大小和可塑性。我们通过光遗传学重新激活婴儿时期复杂经历中标记的齿状回植入细胞,在神经正常小鼠中挽救了同样明显被遗忘的婴儿记忆。此外,我们通过人工更新记忆印记,永久性地恢复了失去的婴儿记忆,证明婴儿健忘症是一个可逆的过程。我们的研究结果表明,婴儿健忘症不仅是由于植入物表达的可逆检索缺陷,而且发育过程中的免疫激活调节先天和可逆的遗忘开关,从而决定是否会发生婴儿健忘主义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


