Photoelectrochemical asymmetric dehydrogenative [2 + 2] cycloaddition between C–C single and double bonds via the activation of two C(sp3)–H bonds
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 2
Abstract
The efficient generation of high structural complexity, which correlates with the number of stereocentres, is an important objective in organic synthesis. Ideally, from a perspective of economy and sustainability, the conversion should include the direct functionalization of unactivated C(sp3)–H bonds. Here we introduce a methodology that enables the generation of complex cyclobutanes with up to four consecutive stereocentres, including all-carbon quaternary stereocentres, from a direct reaction of C–C single bonds with C=C double bonds. The asymmetric photoelectrocatalysis combines photocatalysis, electrochemical redox catalysis and asymmetric catalysis. It avoids the use of chemical oxidants, exhibits excellent enantioselectivity and diastereoselectivity, reveals high functional group compatibility, and also succeeds in the simultaneous conversion of two C(sp3)–H bonds into consecutive carbon stereocentres. This work demonstrates the power of combining electrochemistry with photochemistry and asymmetric catalysis to generate complex structures in an economic and sustainable fashion. Asymmetric catalytic photoelectrochemical reactions for the construction of complex compounds are underdeveloped. Now, merging photoelectrochemistry with asymmetric catalysis has enabled the dehydrogenative [2 + 2] photocycloaddition between alkyl ketones and alkenes affording enantioenriched cyclobutanes.
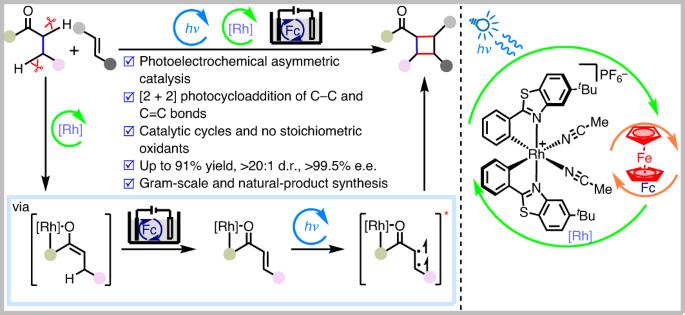
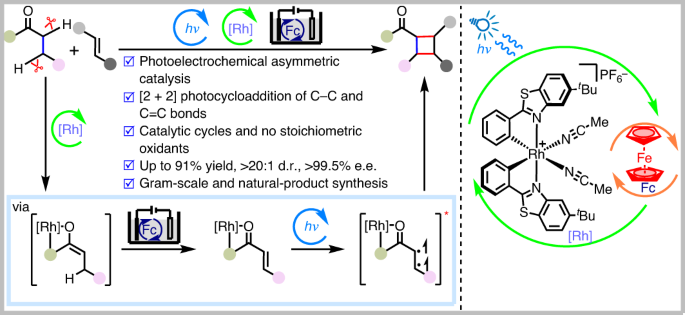
光电化学不对称脱氢[2 + 2] 通过激活两个C(sp3)–H键在C–C单键和双键之间进行环加成
高效产生与立体中心数量相关的高结构复杂性是有机合成的一个重要目标。理想情况下,从经济性和可持续性的角度来看,转化应包括未活化的C(sp3)–H键的直接功能化。在这里,我们介绍了一种方法,该方法能够通过C–C单键与C=C双键的直接反应生成具有多达四个连续立体中心的复杂环丁烷,包括所有碳季立体中心。不对称光电催化结合了光催化、电化学氧化还原催化和不对称催化。它避免了使用化学氧化剂,表现出优异的对映选择性和非对映选择性,显示出高官能团相容性,还成功地将两个C(sp3)–H键同时转化为连续的碳立体中心。这项工作证明了电化学与光化学和不对称催化相结合以经济和可持续的方式产生复杂结构的力量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


