Age-related dysregulation of homeostatic control in neuronal microcircuits
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Neuronal homeostasis prevents hyperactivity and hypoactivity. Age-related hyperactivity suggests homeostasis may be dysregulated in later life. However, plasticity mechanisms preventing age-related hyperactivity and their efficacy in later life are unclear. We identify the adult cortical plasticity response to elevated activity driven by sensory overstimulation, then test how plasticity changes with age. We use in vivo two-photon imaging of calcium-mediated cellular/synaptic activity, electrophysiology and c-Fos-activity tagging to show control of neuronal activity is dysregulated in the visual cortex in late adulthood. Specifically, in young adult cortex, mGluR5-dependent population-wide excitatory synaptic weakening and inhibitory synaptogenesis reduce cortical activity following overstimulation. In later life, these mechanisms are downregulated, so that overstimulation results in synaptic strengthening and elevated activity. We also find overstimulation disrupts cognition in older but not younger animals. We propose that specific plasticity mechanisms fail in later life dysregulating neuronal microcircuit homeostasis and that the age-related response to overstimulation can impact cognitive performance. Radulescu et al. show that homeostatic mechanisms that reduce cortical activity following overstimulation are dysregulated later in life, such that overstimulation results in synaptic strengthening, elevated activity and cognitive impairment.
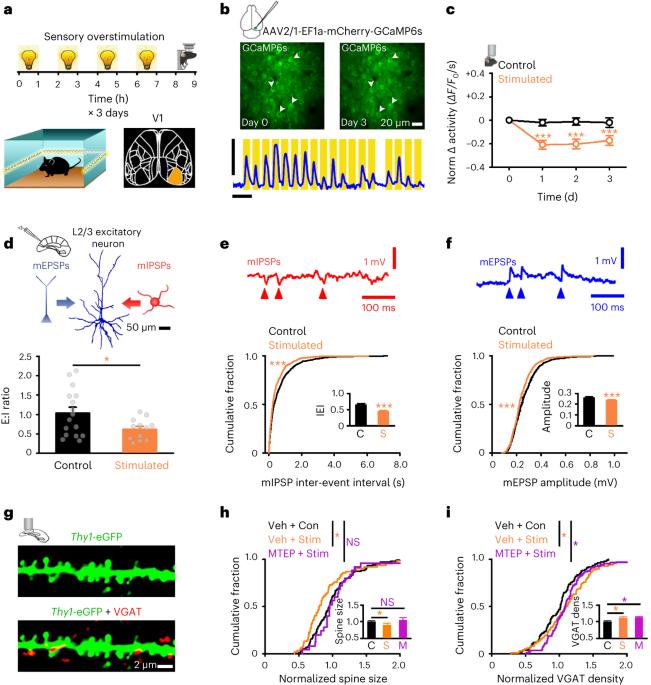
神经元微循环中与年龄相关的稳态控制失调。
神经元稳态可防止多动和低活动。与年龄相关的多动症表明,在以后的生活中,体内平衡可能失调。然而,预防与年龄相关的多动症的可塑性机制及其在以后生活中的功效尚不清楚。我们确定了成人皮层对感觉过度刺激引起的活动增加的可塑性反应,然后测试可塑性如何随年龄变化。我们使用钙介导的细胞/突触活性的体内双光子成像、电生理学和c-Fos-活性标记来显示成年晚期视觉皮层对神经元活性的控制失调。具体而言,在年轻成年皮层中,mGluR5依赖的群体范围兴奋性突触减弱和抑制性突触发生降低了过度刺激后的皮层活动。在以后的生活中,这些机制被下调,因此过度刺激导致突触增强和活动增加。我们还发现,过度刺激会扰乱老年动物的认知,但不会破坏年轻动物的认知。我们提出,特定的可塑性机制在晚年失调神经元微电路稳态中失败,并且与年龄相关的过度刺激反应会影响认知表现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


