Nirmatrelvir/ritonavir use in pregnant women with SARS-CoV-2 Omicron infection: a target trial emulation
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
To date, there is a lack of randomized trial data examining the use of the antiviral nirmatrelvir/ritonavir in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected pregnant persons. This target trial emulation study aimed to address this gap by evaluating the use of nirmatrelvir/ritonavir in nonhospitalized pregnant women with symptomatic SARS-CoV-2 Omicron variant infection. Among patients diagnosed between 16 March 2022 and 5 February 2023, exposure was defined as outpatient nirmatrelvir/ritonavir treatment within 5 days of symptom onset or coronavirus disease 2019 (COVID-19) diagnosis. Primary outcomes were maternal morbidity and mortality index (MMMI), all-cause maternal death and COVID-19-related hospitalization, while secondary outcomes were individual components of MMMI, preterm birth, stillbirth, neonatal death and cesarean section. One-to-ten propensity-score matching was conducted between nirmatrelvir/ritonavir users and nonusers, followed by cloning, censoring and weighting. Overall, 211 pregnant women on nirmatrelvir/ritonavir and 1,998 nonusers were included. Nirmatrelvir/ritonavir treatment was associated with reduced 28-day MMMI risk (absolute risk reduction (ARR) = 1.47%, 95% confidence interval (CI) = 0.21–2.34%) but not 28-days COVID-19-related hospitalization (ARR = −0.09%, 95% CI = −1.08% to 0.71%). Nirmatrelvir/ritonavir treatment was also associated with reduced risks of cesarean section (ARR = 1.58%, 95% CI = 0.85–2.39%) and preterm birth (ARR = 2.70%, 95% CI = 0.98–5.31%). No events of maternal or neonatal death or stillbirth were recorded. The findings suggest that nirmatrelvir/ritonavir is an effective treatment in symptomatic pregnant women with SARS-CoV-2 Omicron variant infection. Analysis of electronic health records of nirmatrelvir/ritonavir use in pregnant women shows that the treatment is associated with a lower risk of pregnancy-related adverse outcomes, including maternal morbidity, premature birth and cesarean section.
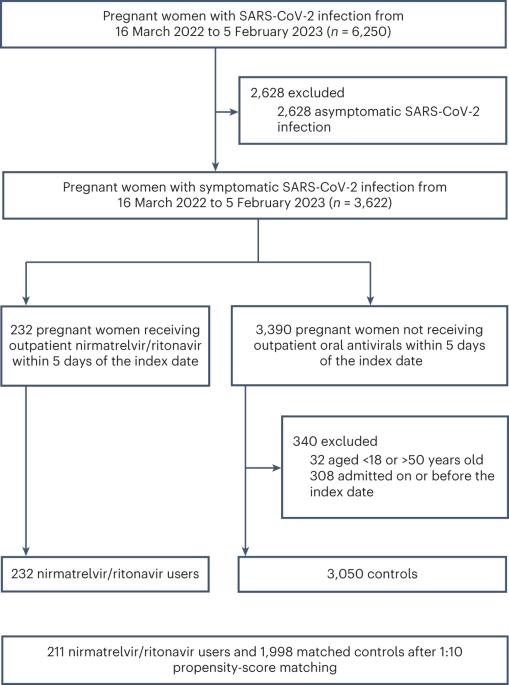
尼马特雷韦/利托那韦在严重急性呼吸系统综合征冠状病毒2型奥密克戎感染孕妇中的应用:一项靶向试验模拟。
到目前为止,缺乏随机试验数据来检查抗病毒药物尼马特雷韦/利托那韦在严重急性呼吸系统综合征冠状病毒2型感染孕妇中的使用情况。这项靶向试验模拟研究旨在通过评估在有症状的严重急性呼吸系统综合征冠状病毒2型奥密克戎变异株感染的非住院孕妇中使用尼马特雷韦/利托那韦来解决这一差距。在2022年3月16日至2023年2月5日期间确诊的患者中,暴露被定义为在症状出现或新冠肺炎确诊后五天内门诊接受尼马特雷韦/利托那韦治疗。主要结果是孕产妇发病率和死亡率指数(MMMI)、全因孕产妇死亡和COVID-19相关住院治疗,而次要结果是MMMI、早产、死产、新生儿死亡和剖腹产的单个组成部分。在尼马特雷韦/利托那韦使用者和非使用者之间进行1至10分倾向评分匹配;然后是克隆、审查和加权。总体而言,211名服用尼马特雷韦/利托那韦的孕妇和1998名非使用者被纳入研究。尼马特雷韦/利托那韦治疗与28天MMMI风险降低相关(绝对风险降低[ARR] = 1.47%,95%CI = 0.21%-2.34%);但不包括与COVID-19相关的28天住院治疗(ARR = -0.09%,95%CI = -1.08%-0.71%)。尼马特雷韦/利托那韦治疗也与降低剖宫产风险有关(ARR = 1.58%,95%CI = 0.85%-2.39%);和早产(ARR = 2.70%,95%CI = 0.98%-5.31%)。未记录到孕产妇或新生儿死亡或死产事件。研究结果表明,尼马特雷韦/利托那韦是治疗严重急性呼吸系统综合征冠状病毒2型奥密克戎变异株感染的有症状孕妇的有效方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


