Carbon-to-nitrogen single-atom transmutation of azaarenes
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
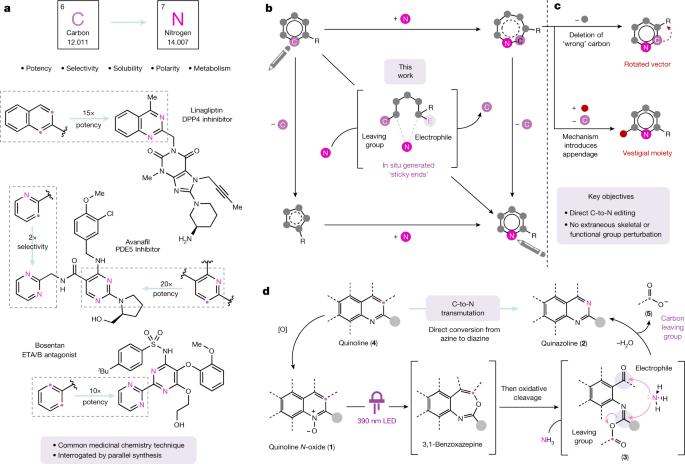
When searching for the ideal molecule to fill a particular functional role (for example, a medicine), the difference between success and failure can often come down to a single atom1. Replacing an aromatic carbon atom with a nitrogen atom would be enabling in the discovery of potential medicines2, but only indirect means exist to make such C-to-N transmutations, typically by parallel synthesis3. Here, we report a transformation that enables the direct conversion of a heteroaromatic carbon atom into a nitrogen atom, turning quinolines into quinazolines. Oxidative restructuring of the parent azaarene gives a ring-opened intermediate bearing electrophilic sites primed for ring reclosure and expulsion of a carbon-based leaving group. Such a ‘sticky end’ approach subverts existing atom insertion–deletion approaches and as a result avoids skeleton-rotation and substituent-perturbation pitfalls common in stepwise skeletal editing. We show a broad scope of quinolines and related azaarenes, all of which can be converted into the corresponding quinazolines by replacement of the C3 carbon with a nitrogen atom. Mechanistic experiments support the critical role of the activated intermediate and indicate a more general strategy for the development of C-to-N transmutation reactions. A new type of transformation converting a heteroaromatic carbon atom into a nitrogen atom, turning quinolines into quinazolines to enable manipulation of molecular properties, is reported.
氮杂烯烃的碳-氮单原子嬗变
在寻找能发挥特定功能作用的理想分子(例如药物)时,成功与失败往往只取决于一个原子1。用氮原子取代芳香族碳原子将有助于发现潜在的药物2,但目前只有间接的方法 进行这种 C-N 转化,通常是通过平行合成3。在此,我们报告了一种能将杂芳香族碳原子直接转化为氮原子,从而将喹啉类化合物转化为喹唑啉类化合物的转化方法。母体氮杂环炔的氧化重组产生了一个开环中间体,该中间体具有亲电位点,可使环重新闭合并释放出碳基离去基团。这种 "粘端 "方法颠覆了现有的原子插入-删除方法,从而避免了逐步骨架编辑中常见的骨架旋转和取代基扰动陷阱。我们展示了范围广泛的喹啉类和相关氮杂烯类化合物,通过用氮原子替换 C3 碳,所有这些化合物都可以转化为相应的喹唑啉类化合物。机理实验证明了活化中间体的关键作用,并为 C-N 变换反应的发展指明了更普遍的策略。报告中介绍了一种将杂芳族碳原子转化为氮原子的新型转化方法,这种方法可将喹啉类化合物转化为喹唑啉类化合物,从而实现对分子性质的操纵。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


