Developing and Optimizing a Quench-Crystallization Operation in Drug Substance Manufacturing
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
We describe the development of a triphasic quench-crystallization operation to isolate an intermediate in the manufacture of the sotorasib drug substance. Using this case study, unique aspects of the quench-crystallization operation are highlighted and contrasted with the development of more commonly discussed cooling/antisolvent crystallization operations. A workflow for exploring and developing complex, reactive crystallization operations for commercial drug substance manufacture is proposed and illustrated. Within this framework, the utility of applying process analytical technology (PAT) is demonstrated.
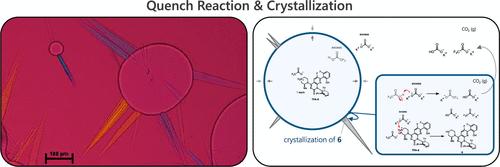
原料药生产中淬火结晶操作的开发与优化
我们描述了一个三相淬火结晶操作的发展,以分离中间体在制造sotorasib原料药。通过本案例研究,强调了淬火结晶操作的独特方面,并与更常讨论的冷却/抗溶剂结晶操作的发展进行了对比。提出并说明了探索和开发用于商业原料药生产的复杂反应结晶操作的工作流程。在此框架内,演示了应用过程分析技术(PAT)的效用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


