C–H Arylation in the Formation of a Complex Pyrrolopyridine, the Commercial Synthesis of the Potent JAK2 Inhibitor, BMS-911543
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 14
Abstract
The development of an improved short and efficient commercial synthesis of the JAK2 inhibitor, a complex pyrrolopyridine, BMS-911543, is described. During the discovery and development of this synthesis, a Pd-catalyzed C–H functionalization was invented which enabled the rapid union of the key pyrrole and imidazole fragments. The synthesis of this complex, nitrogen-rich heterocycle was accomplished in only six steps (longest linear sequence) from readily available materials.
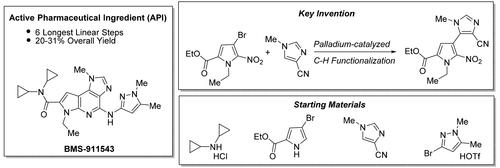
C-H芳基化在络合物吡罗吡啶形成中的作用,强效JAK2抑制剂的商业合成,BMS-911543
本文描述了一种改进的JAK2抑制剂,一种配合吡罗吡啶BMS-911543的短时间高效商业合成方法的发展。在该合成的发现和开发过程中,发明了一种pd催化的C-H功能化方法,使关键吡咯和咪唑片段能够快速结合。这种复杂的富氮杂环的合成只需要六个步骤(最长的线性序列)就可以从现成的材料中完成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


