Site‐Selective Trifluoromethylation Reactions of Oligopeptides
IF 2.8
4区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 16
Abstract
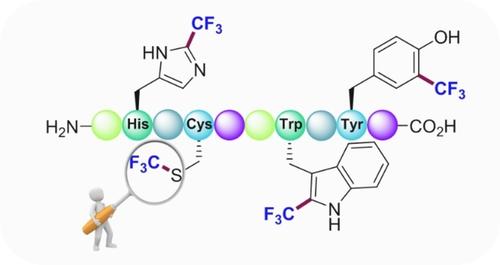
Site‐selective chemical modifications that target proteinogenic amino acid residues complement the methods entailing genetic manipulation, thereby allowing straightforward and rapid access to engineered proteins. The incorporation of the trifluoromethyl group into amino acids within a peptide sequence results in relevant peptidomimetics with unique biomedicinal properties. As a result, the last decade has witnessed the development of a powerful set of protocols toward the selective trifluoromethylation of small‐to‐medium size peptides and proteins in a late‐stage fashion. This minireview seeks to highlight those particularly compelling cases published in the last years.
寡肽的位点选择性三氟甲基化反应
靶向蛋白质生成氨基酸残基的位点选择性化学修饰补充了涉及基因操作的方法,从而允许直接和快速地获得工程蛋白。将三氟甲基结合到肽序列内的氨基酸中,产生具有独特生物医学特性的相关拟肽物。因此,过去十年见证了一套强大的方案的发展,以晚期的方式选择性地三氟甲基化小到中等大小的肽和蛋白质。这篇小型综述旨在突出那些在过去几年发表的特别引人注目的案例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Asian Journal of Organic Chemistry
CHEMISTRY, ORGANIC-
CiteScore
4.70
自引率
3.70%
发文量
372
期刊介绍:
Organic chemistry is the fundamental science that stands at the heart of chemistry, biology, and materials science. Research in these areas is vigorous and truly international, with three major regions making almost equal contributions: America, Europe and Asia. Asia now has its own top international organic chemistry journal—the Asian Journal of Organic Chemistry (AsianJOC)
The AsianJOC is designed to be a top-ranked international research journal and publishes primary research as well as critical secondary information from authors across the world. The journal covers organic chemistry in its entirety. Authors and readers come from academia, the chemical industry, and government laboratories.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


