Modified response evaluation criteria in solid tumors: A better response evaluation criteria for patients with non-squamous non-small cell lung cancer after bevacizumab treatment
Abstract
Aim
Cavitation of lesions is common in non-squamous non-small cell lung cancer (non-squamous-NSCLC) patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFRIs). However, traditional response evaluation criteria in solid tumors (RECIST) do not take cavitation into consideration and may no longer be accurate for potentially reflecting the real clinical efficacy of anti-vessel growth therapy. Here, we aimed to optimize the traditional RECIST version 1.1 by adding cavitation into the evaluation criteria.
Methods
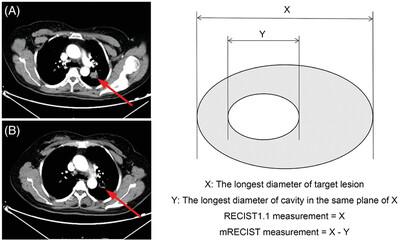
We performed a post-hoc radiologic review of 517 patients in a phase III clinical trial of bevacizumab biosimilar (SIBP04) combined with chemotherapy for the treatment of non-squamous NSCLC. Tumor responses were assessed by RECIST1.1 and mRECIST criteria (modified RECIST, a novel alternate method where the longest diameter of the cavity was subtracted from the overall longest diameter of that lesion to measure target lesions), respectively, and correlated with clinical outcomes.
Results
Cavitations of pulmonary lesions were seen in nine (2%) patients at baseline, and 97 (19%) during treatment. The use of mRECIST resulted in an alteration of the response category. For patients with post-therapy cavitation, the objective response rate was 56% using RECIST1.1 and 67% by mRECIST. In addition, the survival rates between partial response, stable disease, and progressive disease when the mRECIST was applied were significantly different (p < 0.05), while RECIST1.1 failed to show survival differences (p = 0.218).
Conclusion
For patients with post-therapy cavitation, mRECIST exhibited higher predictability of survival than RECIST1.1. Response assessment might be improved by incorporating cavitation into assessment, potentially altering outcomes of key clinical efficacy parameters.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


