A Thiol-Free Route to Alkyl Aryl Thioethers
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Most existing methods for the synthesis of alkyl aryl thioethers require the use of mercaptans as the starting materials, which comes with practical limitations. Reactions of diaryliodonium salts with xanthate salts, easily prepared from the corresponding alcohols and CS2, under the developed conditions represent an operationally simple, thiol-free method for the synthesis of these valuable compounds. The protocol features high functional group tolerance and can be applied to the late-stage C–H functionalization and for the introduction of a CD3S group.
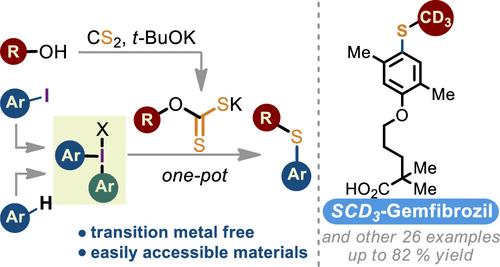
无硫醇合成烷基芳基硫醚的途径
大多数现有的合成烷基芳基硫醚的方法都需要使用硫醇作为起始原料,这具有实际的局限性。在此条件下,二芳基硫鎓盐与黄药盐的反应是合成这些有价值化合物的一种操作简单、无硫醇的方法。该方案具有高官能团耐受性,可应用于后期C-H功能化和CD3S基团的引入。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


