Risk heterogeneity of bullous pemphigoid among dipeptidyl peptidase-4 inhibitors: A population-based cohort study using Japanese Latter-Stage Elderly Healthcare Database
Abstract
Aims/Introduction
Although the association between dipeptidyl peptidase-4 (DPP-4) inhibitors and bullous pemphigoid (BP) has begun to be established, some studies have suggested there are risk differences among DPP-4 inhibitors. We conducted a population-based cohort study to examine the risk differences.
Materials and Methods
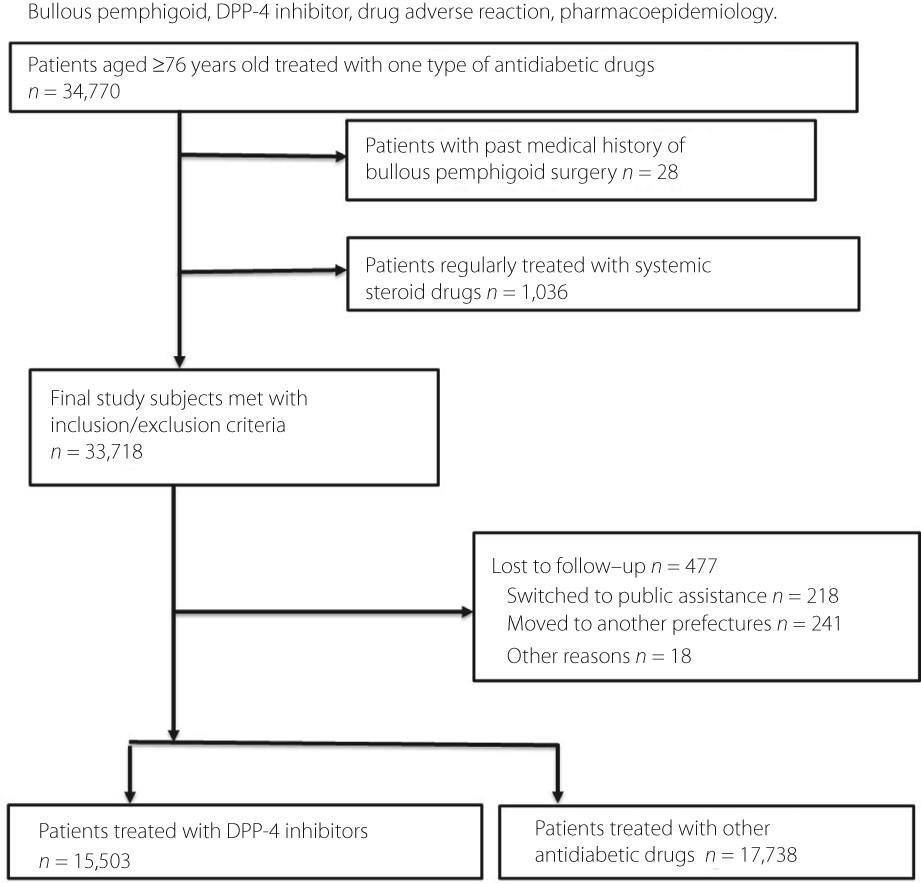
Using the claims databases of the Fukuoka Prefecture Wide-Area Association of Latter-Stage Elderly Healthcare between April 1, 2013 and March 31, 2017, we conducted a retrospective cohort study to compare patients receiving one DPP-4 inhibitor with those who were prescribed another antidiabetic drug. The primary outcome was an adjusted hazard ratio (HR) of the development of bullous pemphigoid during a 3-year follow-up. The secondary outcome was the development of BP requiring systemic steroids immediately after the diagnosis. These were estimated using Cox proportional hazards regression models.
Results
The study comprised 33,241 patients, of which 0.26% (n = 88) developed bullous pemphigoid during follow-up. The percentages of patients with bullous pemphigoid who required immediate systemic steroid treatment was 0.11% (n = 37). We analyzed four DPP-4 inhibitors: sitagliptin, vildagliptin, alogliptin, and linagliptin. Vildagliptin and linagliptin raised the risk of BP significantly (primary outcome, vildagliptin, HR 2.411 [95% confidence interval (CI) 1.325–4.387], linagliptin, HR 2.550 [95% CI 1.266–5.136], secondary outcome, vildagliptin HR 3.616 [95% CI 1.495–8.745], linagliptin HR 3.556 [95% CI 1.262–10.024]). A statistically significant risk elevation was not observed with sitagliptin and alogliptin (primary outcome, sitagliptin, HR 0.911 [95% CI 0.508–1.635], alogliptin, HR 1.600 [95% CI 0.714–3.584], secondary outcome, sitagliptin, HR 1.192 [95% CI 0.475–2.992], alogliptin, HR 2.007 [95% CI 0.571–7.053]).
Conclusions
Not all the DPP-4 inhibitors could induce bullous pemphigoid significantly. Therefore, the association warrants further investigations before generalization.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


